Chemistry Reference
In-Depth Information
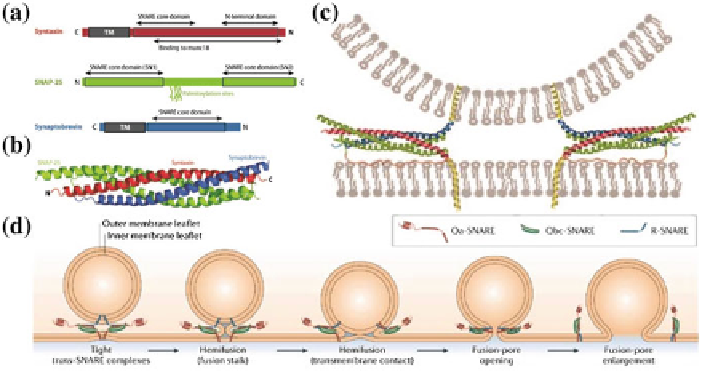
Fig. 3.1
SNARE protein complex and SNARE-mediated membrane fusion.
a
Schematic of the
three components of the SNARE complex: Syntaxin, SNAP-25 and Synaptobrevin. Syntaxin and
Synaptobrevin have transmembrane domains (TM) at their C terminals. The TM domains embed
into the lipid bilayer. SNAP-25 and Syntaxin are present on the plasma membrane. Synaptobrevin
is present on the vesicle.
b
The coil-coil structure of the SNARE proteins. The coiling proceeds
from the C terminals towards the N terminals.
c
When the proteins coil, the two lipid bilayers of
the plasma membrane and the synaptic vesicle are pulled close together.
d
Once the membranes are
close enough, the lipid bilayers fuse through a sequence of events such as hemifusion, formation
of a fusion pore and finally complete fusion (Figures reproduced with permission from [
1
,
2
])
Another key player in the fusion process is synaptotagmin-1, the
Ca
2
+
-trigger for
fast exocytosis in neurons. It promotes the interaction of SNARE proteins between
the synaptic vesicles and the plasma membrane, which results in membrane fusion
and release of neurotransmitter. Synaptotagmin-1 is a 65 kDa integral membrane
protein present on synaptic vesicles, which functions as a major
Ca
2
+
-sensor for
neuronal exocytosis [
3
,
4
]. As shown in the cartoon sketch of Fig.
3.2
, it contains a
single transmembrane domain followed by a large cytoplasmic domain consisting of
a 61 residue unstructured linker and tandemC2-type phospholipid and
Ca
2
+
-binding
domains. The C2-domains, called the C2A and C2B domain, bind 2 and 3
Ca
2
+
ions,
respectively, with low affinity (60
M-1 mM) [
5
,
6
]. The C2-domains interact with
membranes containing anionic lipids and with SNARE proteins. Synaptotagmin-1
can bind to anionic lipids both in the absence of
Ca
2
+
via a so-called polybasic patch
consisting of four lysines located on the C2B domain [
7
-
9
] and in the presence of
Ca
2
+
via its
Ca
2
+
-binding sites [
5
,
8
-
13
]. Anionic lipids such as phosphoserine (PS)
and phosphatidylinositol 4,5-biphosphate (
Pi
µ
P
2
) complete the
Ca
2
+
-binding
sites of the C2-domains and thereby increase the affinity of synaptotagmin-1 for
Ca
2
+
-binding [
5
,
7
,
8
,
14
].
However, while the zippering process of the SNARE proteins is reasonably well
established, the role of synaptotagmin-1 in the membrane fusion process is not fully
clear. The exact mechanism by which it acts as a
Ca
2
+
trigger is poorly understood.
The sequence of events in the membrane fusion is problematic too. If the SNARES
(
4
,
5
)