Game Development Reference
In-Depth Information
Figure 14-3.
When an atom absorbs energy, one of its electrons can move to a higher energy state.
Figure 14-4.
When an electron transitions to a lower energy state, it emits a photon.
Other energy level transitions would be possible with the hydrogen atom. If an electron
transitioned from the third excited energy state to the second, a photon would be released with
an energy level of 0.65
eV
. You have seen the effect of electron energy level transitions many
times. An incandescent light bulb works because the atoms in the metal filament are transi-
tioning energy states and giving off photons.
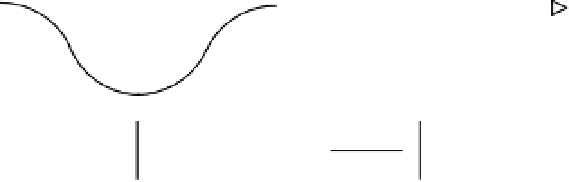
Photons move about at the speed of light. The motion of a photon follows a wavelike pattern.
As shown in Figure 14-5, every photon will have a
wavelength
associated with it that is the
distance between successive peaks of the wave pattern of the photon.
Figure 14-5.
Every photon will have a wavelength.