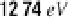Game Development Reference
In-Depth Information
Electrons have a negative electric charge. Protons have a positive electric charge. Because
of the charge difference, there is an electrostatic force that pulls the electrons towards the
nucleus. Balancing the electrostatic force is the centripetal force of the electrons as they travel
around the nucleus. The radius of the electron orbit is determined by the energy stored by the
electron, also known as the
energy level
of the electron. The orbits of electrons at lower energy
levels will be closer to the nucleus. This description is a fairly simplified view of the structure of
an atom, but it is useful to make the connection between electron energy levels and the corre-
sponding electron orbit.
When an atom absorbs energy, whether it's from heat, light, electricity, or some other
energy source, some of the electrons in lower energy states will transition into higher energy
states and will acquire new orbits further away from the nucleus. These higher energy levels are
known as
excited energy states
. It turns out that electrons can't be at just any old energy level.
Instead, electrons can only exist at discrete energy levels. Another way to think about this situ-
ation is that electrons can only travel in certain discrete orbits around the nucleus.
As an example, Figure 14-2 shows the energy levels available to the electrons in a hydrogen
atom. In Figure 14-2 the electron energy levels are represented as a column of horizontal lines.
Electron energy levels are usually expressed in terms of electron volts, or
eV
, which is a very
small amount of energy. One
eV
is equal to 1.602
e
- 19
J
. The lowest energy state is referred to
as the
ground state
. The excited energy levels are expressed relative to the ground state.
Figure 14-2.
Energy levels for hydrogen atom electrons
Atomic hydrogen has three excited energy states. If a hydrogen atom absorbs 10.2
eV
of
energy, as shown in Figure 14-3, then one of its electrons can transition from the ground energy
state to the first excited energy state. If the atom absorbed 12.09
eV
, then an electron could
transition from the ground state to the second excited energy state. Electrons can also move
from one excited energy state to a higher one.
Just as electrons can transition from a lower energy state to a higher energy state, they can
go the other way as well. In fact, excited electrons will naturally tend to return to lower energy
states. In order for an electron to move to a lower energy state, it must release some of its energy
as a
photon
—a particle of light. The amount of energy released, and the energy of the resulting
photon, is equal to the energy difference between the two states. In Figure 14-4, a hydrogen
atom electron is transitioning from the first excited state to the ground state. In order to do this,
the electron emits a photon with an energy level of 10.2
eV
.