Biomedical Engineering Reference
In-Depth Information
OH
O
O
O
O
O
O
O
O
H
N
H
N
H
N
H
N
H
N
H
N
H
N
N
H
N
H
N
H
N
H
N
H
N
H
N
H
NH
2
O
O
O
O
O
O
O
NH
NH
NH
2
NH
NH
NH
38
NH
2
NH
2
NH
2
H
2
N
H
2
N
H
2
N
H
2
N
N
N
N
N
39
H
O
O
H
S
S
S
N
H
N
H
N
H
N
H
N
H
N
H
N
H
N
H
Fig. 13 The cationic peptide 38 and the positively charged tetraguanidinium moiety 39 recognize
p53 via four carboxylates at the protein surface
2.2 Cationic Ligands
Giralt synthesized protein surface-binding molecules, including the cationic pep-
tide 38 or the non-peptidic tetraguanidinium ligand 39, which are illustrated in
Fig.
13
, for the recognition of the tetramerization domain of p53 via four glutamate
moieties on the protein surface [
36
]. This protein is an important transcription
factor for the regulation of apoptosis and cell division and as such is an interesting
target for the development of novel drugs against cancer. The binding constant for
38 and similar peptides towards p53 was determined to be
K
10
5
M
1
, and the
¼
10
4
M
1
.
Schmuck identified highly efficient tetravalent inhibitors of
tetraguanidinium derivative 39 binds with
K
¼
2
-tryptase via an on-
bead fluorescence screening of a ligand library, 40, containing 216 (6
3
) members
(Fig.
14
)[
37
]. The tryptase is a human serine protease, which plays a decisive role
in the pathogenesis of asthma and other allergic and inflammatory disorders. In its
active tetrameric form, the enzyme features a central pore, in which four active sites
are located. A cluster of negatively charged amino acids is found at the entrance to
this channel. With the help of the library it could be shown that tetrameric ligands
with complementary charge are able to block the entrance to this channel and thus
prevent the substrate from accessing the active sites. As a consequence, the enzyme
is inhibited non-competitively. The best inhibitors combined aromatic and cationic
amino acids and the overall best sequence, with an excellent nanomolar activity
(
K
i
¼
b
170 nM), featured the amino acid sequence Arg-Trp-Lys (from AA
1
to AA
3
).
Furthermore, a clear multivalency effect was observed: the analogous one-armed
ligand was less active by a factor of 1,800 (
K
i
¼
306
M). Finally, the thus
m
identified inhibitors were selective for
-tryptase and did not influence similar
enzymes, such as trypsin or chymotrypsin.
In conclusion, multivalent protein-ligands have proven to be highly efficient in
recognizing protein surfaces. Up till now these recognition events were mainly
based on charge-charge interactions, which allowed the design of ligands featuring
b
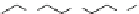




































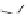




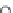





















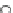















































Search WWH ::

Custom Search