Biomedical Engineering Reference
In-Depth Information
NH
NH
2
NH
2
NH
HN
H
2
N
NH
2
NH
2
NH
2
6
7
8
H
NH HN
H
N
NH
HN
HN
NH
HN
HN
NH
NH
NH
H
N
9
10
11
Fig. 2 Linear polyamines putrescine 6, spermidine 7 and spermine 8 (
top
) and exemplary
macrocyclic polyamine receptors 9-11 (
bottom
)
NH
H
H
N
N
HN
HN
N
HN
NH
H
N
N
NH
12
13
Fig. 3 Macrocyclic polyamine receptor 12 with two naphthalene bridges (
left
) and phenanthroline
linked receptor 13 (
right
)
10
3
M
1
). Finally, spermine forms even stronger 1:1
complexes with ATP, with a binding constant of 10
4
M
1
. Obviously complex
formation becomes stronger the greater the number of charge-charge interactions it
is possible to form.
Not surprisingly, the first artificial nucleotide receptors, developed by Kimura,
were 15- to 18-membered macrocyclic polyamines—some exemplary representatives
are shown in Fig.
2
(9-11)[
3
]. By studying their binding properties towards phos-
phate, AMP, ADP, and ATP via polarographic and NMR measurements in buffered
water at neutral pH, binding constants of up to 4
complexes with ADP (
K
¼
10
6
M
1
could be observed.
Again, ATP was bound strongest followed by ADP and AMP. Phosphate on its own
was bound more weakly than AMP by a factor of 10-100, although their charge is the
same. This effect was attributed to additional hydrogen bonds between the nucleobase
and the receptor as indicated by NMR. By comparing spermine with its cyclic analog
10 it could also be shown that the macrocycle binds more strongly to nucleotides by
approximately two orders of magnitude. Furthermore, only polyamines which are
capable of incorporating at least three charges at neutral pH formed stable complexes.
The next generation of receptors incorporated aromatic rings into the
macrocycle. Lehn developed the nucleotide host 12 depicted in Fig.
3
, which is
able to bind to nucleotides in buffered water at pH 6 with binding constants of up to
2
10
5
M
1
(ATP, NMR titration) [
4
]. The less charged AMP is bound more
weakly by one order of magnitude. Interestingly the purine-derived nucleotides
AMP and GMP are bound more strongly than the smaller pyrimidine analogs CMP
and UMP by a factor of 10. Lacking the phosphate hinge, the corresponding
nucleosides are more weakly bound by 2-3 orders of magnitude. Thus it can be
concluded that both the stacking between nucleobase and aromatic scaffold as well
















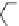










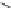

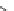






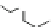
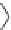
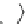































Search WWH ::

Custom Search