Biomedical Engineering Reference
In-Depth Information
N
N
N
H
H
H
N
NH
HN
N
N
H
14
15
Fig. 4 Tweezer receptor 14 with two polyamine side chains linked via a phenanthroline template
(
left
) and receptor 15 with two anthracene units bridged by a polyamine chain
as charge-charge interactions between the polyammonium chains and the phos-
phate part of the nucleotide are essential for efficient binding.
Bencini developed the macrocyclic receptor 13 (Fig.
3
) with a phenanthroline unit
inside the polyamine macrocycle [
5
]. With the help of potentiometric measurements,
the highest affinity was found for ATP with an extremely high binding constant of
3
10
9
M
1
. The corresponding nucleotides TTP, CTP, and GTP were bound less
well by 1-2 orders of magnitude. Furthermore, the artificial host could selectively sense
ATP thanks to fluorescence quenching caused by a photoinduced electron transfer from
an amine group of the receptor to the excited phenanthroline. Concerning the complex
geometry, it could be shown by means of NMR experiments, molecular modeling and a
crystal structure of one of the complexes that the polyamine chain forms a cavity for the
phosphate, enforced by ion pairs and hydrogen bonds, while the nucleobase stacks with
the phenanthroline moiety. A macrocycle analog with reduced ring size and one fewer
amine group lost a huge part of its affinity, i.e., the binding constant to ATP was only
10
6
M
1
—lower by a factor of 3,000. These results demonstrate the importance of an
appropriate mix of attractive interactions with the correct geometric prerequisites
needed for efficient recognition of a given substrate molecule.
Lin designed the tweezer receptor 14 (Fig.
4
) with two polyamine side chains
linked via a phenanthroline scaffold; each arm incorporates a phenyl headgroup [
6
].
The binding constant for ATP was measured via potentiometry to be an excellent
7.9
10
10
M
1
. As expected, NMR and molecular modeling revealed
charge-charge interactions between phosphate and polyammonium side chains
and
p
-stacking between phenanthroline and phenyl groups with the nucleobase.
These results prove that in order to achieve high binding affinity it is not necessary
to make use of cyclic systems: Essentially the combination of several attractive
non-covalent interactions in the correct molecular topology for binding is more
important for the strength of the molecular recognition event.
A similar system (15), also depicted in Fig.
4
, was developed by Garcia-Espa˜a
.
Two anthracene moieties are linked by a polyamine chain [
7
]. As determined by
potentiometry, the binding constant for ATP was also similarly high, being measured
as 10
9
M
1
. ADP and ATP were bound consecutively worse by two and, respectively,
three orders of magnitude. Furthermore, in this work also the effective binding constant
for ATP at neutral pH was determined to be approximately 5
10
5
M
1
. This value
was confirmed with the help of a fluorescence titration. It is noteworthy that the
effective binding constant is lower by more than three orders of magnitude compared
to the highest possible value reported, based on potentiometric measurements.


























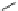




































Search WWH ::

Custom Search