Biomedical Engineering Reference
In-Depth Information
H
O
H
H
O
N
O
H
O
H
N
EtOOC
O
H
S
O
H
N
N
S
Fig. 27 Structural proposal for the complex formed between ligand 25 and TMA maleate
Table 2 Stoichiometry and log
for ligands 25 and 26 with TMA salts of oxalate (DC2),
malonate (DC3), succinate (DC4), glutarate (DC5), and adipate (DC6) in DMSO by UV-Vis
spectroscopy
Ligand
b
DC2
DC3
DC4
DC5
DC6
25
Log
b
7.9
0.2
2.8
0.2
3.41
0.01
3.3
0.2
3.86
0.03
L:DC
1:2
1:1
1:1
1:1
1:1
26
Log
b
3.66
0.03
3.8
0.4
-
-
3.1
0.4
L:DC
1:2
1:1
-
-
1:1
O
R
O
NH
S
O
O
O
HN
R
N
EtOOC
NH
O
O
EtOOC
t
t
(CH
2
)n
S
t
t
S
EtOOC
NH
EtOOC
N
O
HN
R
S
O
NH
R
O
O
O
Fig. 28 Proposal structures for 1:1 and 1:2 complexes between ligand 25 and
a
,
o
-dicarboxylates
the complexation observed for maleate. However, oxalate with a different complex
stoichiometry does not induce marked changes in fluorescence. In conclusion,
ligand 25 is able to distinguish the shortest dicarboxylate from longer dianions.
These results are of much interest because discrimination of dicarboxylates
according to their chain length is not usual [
48
,
49
].
Ligands 25 and 26 can be used as sensors through a second channel: UV-Vis
spectroscopy. Therefore, complexation with the studied dicarboxylates gives rise to
modifications in the UV-Vis spectra, which depend on the complex stoichiometry.




















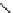










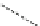




























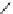
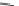



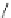












































Search WWH ::

Custom Search