Biomedical Engineering Reference
In-Depth Information
In any case, the observed modifications in fluorescence are more intense than the
corresponding changes in UV.
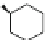
Related racemic ligands with only one thiourea group and a free hydroxyl group,
27 and without ethoxycarbonyl groups, 28 have been also evaluated. Compound 27
brings about a new band at 350 nm upon complexation with dicarboxylates whose
intensity depends on chain length reaching a maximum for DC4. Regarding the
fluorescence channel, CD3 and DC4 cause intermolecular excimer formation with
the corresponding band at 295 nm. The complexes formed with DC2, DC5, and
DC6 present no modifications in the fluorescence spectra.
On the other hand, the studies carried out with ligand 28 reveal that the presence
of the two ethoxycarbonyl groups is essential for the ligand to act as a sensor. In the
absence of these groups the higher flexibility of the ligand precludes both inter and
intramolceular excimer formation [
50
].
EtOOC
NHCSNHR
NHCSNHR
R=
EtOOC
OH
NHCSNHR
27
28
The good results obtained with cyclohexane-based naphthylthiourea ligands
prompted us to synthesize the homochiral series to explore their application in
chiral sensing [
51
]. Thus, ligands 25 and 27 were obtained as pure enantiomers, and
their absolute configurations were determined from the circular dichroism spectra
with the help of the exciton chirality rule [
52
].
EtOOC
EtOOC
R'
R'
R=
EtOOC
EtOOC
NHCSNHR
NHCSNHR
R'=NHCSNHR (-)-
25
R'= OH (-)-
27
R'=NHCSNHR (+)-
25
R'= OH (+)-
27
From the fluorescence titration studies performed with TMA salts of
L
-aspartate,
D
-aspartate,
L
-glutamate, and
D
-glutamate, the complexes' stoichiometry and equili-
brium constants were determined. They follow a similar trend to that previously
described, but the excimer band is observed only for ligands (+)-25 with
D
-aspartate
and (
)-25 with
L
-aspartate, which leads to 1:1 complexes. The R configuration of
D
-
aspartate perfectly fits the complex formed with (+)-25 and induces a conformational
change in the ligand, which places both naphthalene groups close and in an
almost parallel arrangement to give rise to intense excimer emission. A less intense
emission is observed upon the addition of (+)-25 with
L
-aspartate and (
)-25 with
D
-
aspartate as its configuration precludes an effective approach of both naphthyl moieties.
Similar results were obtained upon the addition of
L
- and
D
-glutamate to the
solutions of (+)-25 and (
)-25. However, less intense excimer bands were observed
in these cases. Longer chain length probably leads to a less tight arrangement
compared with that for aspartate.

































Search WWH ::

Custom Search