Biomedical Engineering Reference
In-Depth Information
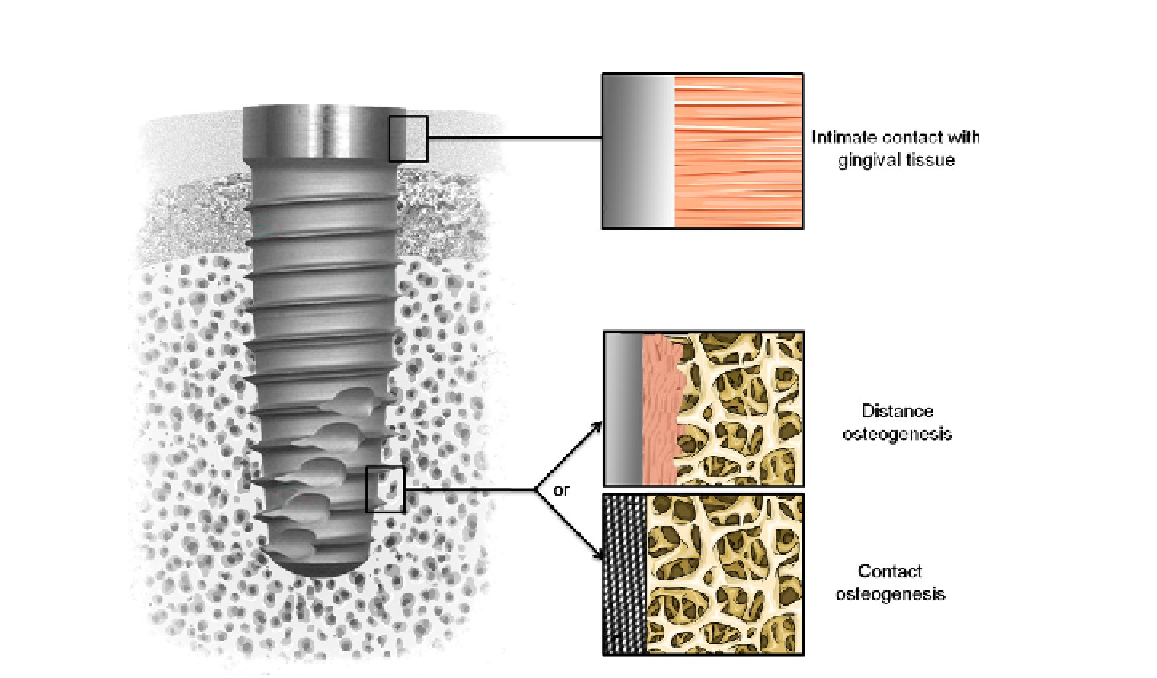
FIGURE 5.1
Tissue integration of dental implant. Note the intimate contact with gingival tissue in the upper part and the
desired contact osteogenesis in the tapered lower part rather than distance osteogenesis.
composition, wettability, and roughness of metal implants surfaces. However, the control of these sur-
face properties at the protein and cell levels, thus in the nanometer range, remains a challenge for
researchers and dental implants manufacturers.
Nanotechnologies may produce surfaces with controlled topography and chemistry that would help
understanding biological interactions and developing novel implant surfaces with predictable tissue-
integrative properties
[2-4]
. Various processing methods derived from the electronic industry such as
lithography, ionic implantation, anodization, radio-frequency plasma treatments may be applied to the
surfaces of dental implants to produce controlled features at the nanometer scale. These surfaces may
then be screened by using high-throughput biological assays
in vitro
. For instance, specific protein
adsorption, cell adhesion, and differentiation of stem cells should be studied in relation to the surface
properties. This approach may define the ideal surface for a specific biological response. Following
in vitro
screening, nanostructured surfaces may then be tested in animal models to validate hypothesis
in a complex
in vivo
environment.
New coating technologies have also been developed for applying hydroxyapatite (HA) and related
calcium phosphates (CaP), the mineral of bone, onto the surface of implants (
Figure 5.2
). Many