Biomedical Engineering Reference
In-Depth Information
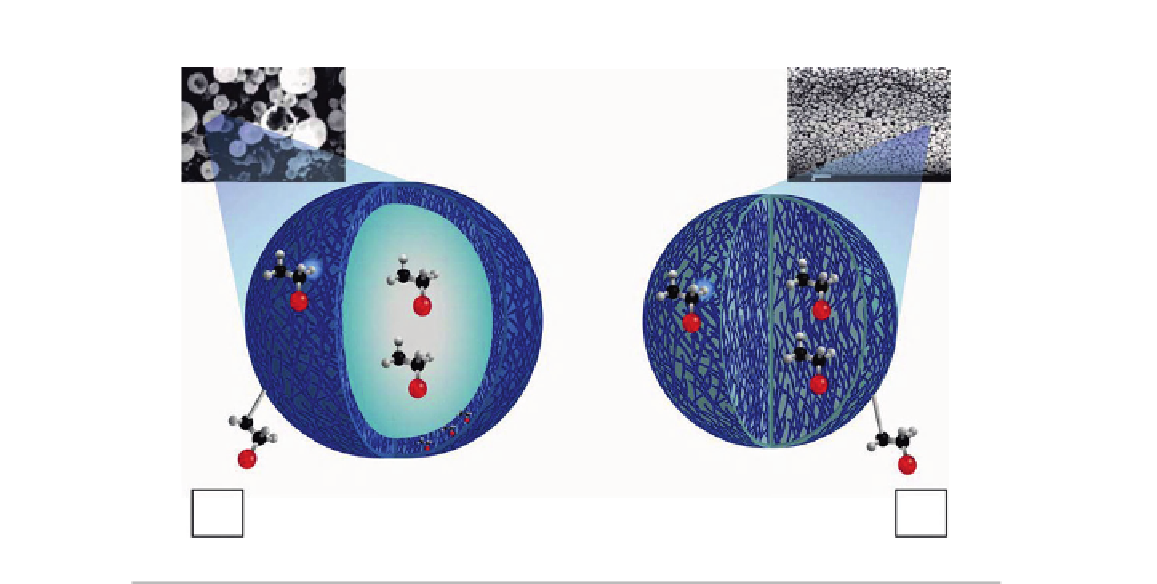
(A)
(B)
FIGURE 23.3
Schematic representation and microphotographies of nanoparticles: (A) nanocapsules and (B) nanospheres.
The “top-down” approach to prepare nanoparticles requires more process steps and, conse-
quently, more control of the preparative variables. The materials are generally dissolved in a sol-
vent to achieve a molecular solution that can be precipitated by solvent changes, e.g., pH,
temperature, and addition of a nonsolvent, thus reducing its solubility. It is possible to prepare
nanoparticles by polymerization of dispersed monomers, but their byproducts may not be
completely biocompatible and toxic residues such as monomers, oligomers, and catalysts may per-
sist. Consequently, their use in dental formulations is restricted. In general, it is preferable to use
preformed materials, especially when polymers are involved. In this sense, another “top-down”
technique is to disperse the molecular solution in an aqueous solution containing stabilizer in order
to obtain a nanoemulsion, which by solvent removal forms nanoparticles. The nanoparticle prepara-
tion methods with high potential in dentistry from preformed polymers can be classified into five
categories: (i) emulsification evaporation, (ii) salting out, (iii) solvent displacement, (iv) emulsifi-
cation diffusion, and (v) spray drying
[50]
. They are summarized in
Figure 23.4
. These techniques
have been broadly discussed elsewhere
[50,52]
. One of the main problems with these techniques is
the poor encapsulation of water-soluble materials, which separate from the organic phase into the
continuous aqueous phase. A double emulsion technique can be used to overcome this drawback.
In the case of solid lipid nanoparticles, the preferred method is high-pressure homogenization
(HPH), in which high-efficiency devices are used to disperse the lipid in a stabilizer solution at
high shear forces, breaking the particles into submicron sizes. This technique has two modalities:
hot and cold homogenization (H-HPH and C-HPH); in both cases, if an active substance is
included, it is necessary to melt the lipid to incorporate the drug (
Figure 23.5
)
[51]
.