Biomedical Engineering Reference
In-Depth Information
and the SNR. The dependence of
TE
on the phase difference con-
strains the CNR
Δφ
to be optimal at
TE
=
T
2
*, which can be shown
by differentiation of Equation 3.13 (Conturo et al. 1990).
A major advantage of the CPD technique for PRF-based
thermometry is its high spatial and temporal resolution (de
Senneville et al. 2007). Typically, fast temperature imaging
can be achieved with GRE acquisitions (Ishihara et al. 1995)
or EPI (Stafford et al. 2004). As stated before, the optimal
TE
for CNR in CPD is at the
T
2
* of the tissue. As a result, standard
GRE acquisitions have a relatively long
TR
, which lowers spatial
and temporal resolution. If higher resolutions are needed, echo
shifts are applied with
TR
<
TE
at an expense of SNR
(de Zwart
et al. 1999). Alternatively, the use of EPI allows for longer
TE
s
without sacrificing resolution or SNR. Techniques using bal-
anced steady-state free-precession (bSSFP) have also been stud-
ied for PRF-based temperature mapping. This is accomplished
by measuring a linear fit along several
TE
s acquired. However,
the phase behavior was found to be highly nonlinear with this
technique, so simple phase to frequency mapping is not feasible
(Mulkern et al. 1998).
Although CPD techniques are advantageous when high
spatial and temporal resolution are required, there are several
well-known limitations of this technique. Intravoxel lipid con-
tamination (Kuroda et al. 1997; de Zwart et al. 1999), inter- and
intra-scan motion (Hynynen et al. 2001), tissue susceptibility
changes (De Poorter 1995), and magnetic field drift (De Poorter
1994) all cause artifacts that are commonly encountered with
CPD techniques.
It is important to remember that the PRF shift is a function
of temperature primarily due to changes in the hydrogen bonds
between water molecules. These hydrogen bonds are absent
for protons in lipid molecules, which are covalently bonded.
Therefore, the temperature sensitivity of lipid is a stronger func-
tion of the macroscopic susceptibility. The presence of lipid can
alter the phase in CPD acquisitions, which, in turn, can affect
temperature estimates. A common practice to address this is to
simply suppress the lipid signal (Kuroda et al. 1997; de Zwart et
al. 1999). Although lipid signal is suppressed, it does not cor-
rect susceptibility effects that lipid can have on the nearby water
molecules. As stated previously, the effect of a mixed water and
lipid environment cannot be handled completely with suppres-
sion because lipid is still physically present in the tissue and can
have an effect on temperature estimation in the voxel via suscep-
tibility effects.
As with most imaging techniques, intra-scan and inter-scan
motion is another common issue that must be addressed with
CPD approaches (Ludemann et al. 2010; Roujol et al. 2010). Intra-
scan motion is motion during the acquisition that results in view
to view k-space errors seen as image blurring and ghosting. To
address this issue, imaging times can be decreased to reduce
intra-scan motion, often at the expense of SNR or resolution.
Inter-scan motion, from organ motion or development of
edema during therapy delivery, is a more difficult problem to
mitigate since images are often subtracted to obtain tempera-
ture estimates (Figure 3.7). In the case of the PRF, motion not
=
|
S
0
||
S
1
|
·
e
i(
φ
0
-
φ
1
)
S
1
*
S
0
=
+ i *
= -
∆φ
arg(
S
1
*
S
0)
=
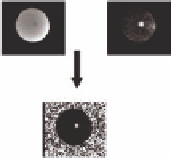
FIGURE 3.5
Complex-phase difference image of a focused ultra-
sound beam in phantom.
The complex phase-difference is calculated
by taking the real and imaging components of the quadrature-detected
image and multiplying the reference baseline image (
S
0
) by the complex
conjugate of the subsequent image (
S
1
). By taking the argument over
all pixels, a map of the Δφ is formed. Note that uniformly distributed
phase occurs in regions with low or no signal. This is often masked by
SNR-based thresholding. Additionally, care must be taken to make sure
the phase is unwrapped in the temporal dimension before converting to
temperature (Equation 3.11).
lipid, and water chemical shifts including intravoxel temperature
variations, pH, magnetic ion concentration, blood-oxygen level
dependent (BOLD) effect,
J
-coupling effects, and magnetic sus-
ceptibility effects (Kuroda 2005). According to a recent review of
MRTI, the “feasibility of absolute temperature imaging has not yet
been established; further detailed investigations will be required
for that” (Kuroda 2005).
Complex phase-difference (CPD) techniques utilizing fast
gradient echo imaging (Ishihara et al. 1995) have been the pre-
ferred method to estimate the PRF shift indirectly. To estimate
the temperature change in a voxel, the difference between the
current phase image, Φ, and the reference image, Φ
ref
, can be
related to the TSC described as (Figure 3.5)
Φ−Φ
π⋅α⋅γ⋅
ref
T
=
(3.11)
2
BTE
⋅
0
where α is the TSC (ppm/°C) and
TE
is the echo-time (ms).
In CPD techniques with sufficient SNR (≥5), the uncertainty
in the CPD image, σ
Δφ
, can be expressed as (Figure 3.6a):
σ≅
σ
2
2
=
(3.12)
φ
ASNR
A
where
A
is the magnitude signal, σ is the noise of the magni-
tude signal (which is assumed to be approximately Gaussian
distributed), and
SNR
A
is the signal-to-noise ratio of the mag-
nitude image (Conturo et al. 1990). A contrast-to-noise ratio
in the phase difference image, CNR
Δφ
, can then be defined by
(Figure 3.6b):
−
TR T
/1
1
−
e
−
TE T
/2*
(3.13)
CNR
∝⋅ ∝⋅
SNR
TE e
⋅ θ⋅
sin( )
φ
A
−
TR T
/1
1
−
e
⋅
cos()
θ
assuming a spoiled gradient-echo acquisition. Note that this is
essentially the product of the
TE
(which is proportional to Δφ)