Biomedical Engineering Reference
In-Depth Information
composition of the liposomes that lead to thermal sensitivity for
drug release have been reviewed elsewhere and will not be dis-
cussed here (Hossann et al. 2007, Lindner et al. 2004, Needham
and Ponce 2007). Yatvin speculated that such liposomes should
greatly increase antitumor effect compared with free drug, if heat-
ing methods were directed specifically toward the tumor. He also
suggested that thermosensitive liposomes might release drug intra-
vascularly, thereby driving drug down its concentration gradient
into the tumor (Yatvin et al. 1978). Although proof of principle was
shown by these authors, the short circulation time of these original
nonsterically stabilized formulations limited their general applica-
bility. Gaber and Papahadjopoulos were the first to formulate a ste-
rically stabilized thermosensitive liposome (Gaber et al. 1995) and
demonstrated enhanced drug release upon reaching the transition
temperature
in vitro
and
in vivo
(Gaber et al. 1996, Wu et al. 1997).
These liposomes and those of Yatvin had two additional limita-
tions, however. The release temperature was too high (43-45°C),
and the rate of release was too slow (30 min)(Gaber et al. 1996).
Dewhirst recognized the limitations of these formulations in dis-
cussions with David Needham in the early 1990s. The performance
characteristics defined by Dewhirst and Needham were used to
develop a thermosensitive liposome that (1) exhibited release in a
more realistic temperature range that could be achieved clinically
(39-42°C) and (2) showed rapid drug release upon reaching the
transition temperature. This latter design characteristic was based
on Dr. Dewhirst's a priori knowledge of typical microvascular flow
velocities in tumors (mm/sec) (Dewhirst et al. 1989), which clearly
dictated that to achieve maximal drug deposition in tumors, release
should occur in seconds, as opposed to 30 min, the release time
associated with the earlier formulations. A rapid release time would
ensure that most drug would be deposited in the tumor before
intravascular liposomes could exit from the tumor.
Needham subsequently reported on a novel pegylated lipo-
some design that incorporated a single chain fatty acid into the
liposome bilayer (Needham et al. 2000, Needham and Dewhirst
2001). This represented a significant departure from earlier for-
mulations that exclusively utilized mixtures of dual chain fatty
acids of different chain lengths to achieve thermal sensitivity.
This liposome, which has been coined the low temperature sensi-
tive liposome, or LTSL, exhibited exactly the design characteris-
tics that were originally stipulated. Lindner and coworkers have
reported on a similar formulation that utilizes synthetic fatty
acids to achieve thermally triggered drug release (Lindner et al.
2004). This group has also optimized the pegylation of such for-
mulations to yield longer circulation times than of the Needham
formulation (Hossann et al. 2007, Li et al. 2010). It remains to be
seen whether a long circulating LTSL is advantageous. The ratio-
nale would be to take advantage of the enhanced vascular per-
meability brought on by hyperthermia, which has been reported
to last up to 4 hr in one tumor model (Kong et al. 2001). Further
studies are needed to ascertain the likelihood that this will per-
form better than the Needham formulation, which exhibits a 2
hr half-life in humans (Poon and Borys 2009). Given that a typi-
cal hyperthermia treatment lasts 30-60 min, the shorter half-life
may be adequate.
0
1
2
3
4
5
%ID/g
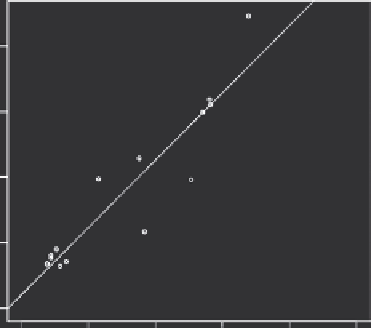
FIGURE 16.3
Data showing that measurement of radiolabelled lipo-
some accumulation in tumors after heating, using a gamma camera,
can be used to accurately determine the accumulation of doxorubicin-
containing liposomes after hyperthermia treatment. These studies
were conducted in rats with R3230Ac mammary carcinomas that were
grown in the flank. (From Kleiter, M. M. et al.,
Clin Cancer Res
, 12,
2006. With permission.)
of vascular destruction and enlarged pore sizes at the margin
of the ablation zone (Monsky et al. 2002). Thermal ablation did
not affect doxorubicin accumulation when it was administered
as free drug in this study.
16.5 Development of thermally
Sensitive Liposomes
The Doxil
•
liposome (a long circulating pegylated doxorubicin-
containing liposome) was originally approved for clinical use
because it exhibits antitumor activity comparable to that of free
drug (O'Brien et al. 2004), but with less cardiac toxicity (Gabizon
et al. 2003, Grenader et al. 2010), which is the main dose limiting
target organ for free doxorubicin. The limitation of the Doxil
•
type
formulation relates to the polyethylene glycol that is added to the
liposome surface to enhance the circulation time. Whereas this
maximizes passive accumulation to tumor via the enhanced per-
meability and retention (EPR) effect, this same polymer coating
reduces the rate at which drug is released once the liposome reaches
the target volume (Landon et al. 2011). The slow drug release rate
greatly reduces the antitumor effect. A number of laboratories have
investigated means to trigger content release from liposomes in
tumors as a means to overcome this limitation. Examples of trig-
gers that take advantage of inherent features of the tumor micro-
environment include sensitivity to acid pH (tumor pH values are
often lower than normal tissue) (Hong and Kim 2011) and relying
on intrinsically enhanced extracellular enzyme activity (such as
metalloproteases) in tumors (Andresen et al. 2010).
In 1978, Yatvin published the first report of a solid phase lipo-
some that underwent a phase transition at 44°C, which led
to enhanced drug release rates (Yatvin et al. 1978). The lipid