Environmental Engineering Reference
In-Depth Information
-2.4
-2.2
-2.6
-2.4
-2.8
-2.6
-3.0
-2.8
-3.2
-3.0
-3.4
-3.2
-3.6
-3.4
-3.8
2.8
3.2
3.6
4.0
4.4
4.8
.8 .2 .6 .0 .4 .8
ln
C
HCl
Fig. 4.44
and
K
when
T
=773 K (
=0.76;
K
=0.003198;
r
2
=0.887)
ln
C
HCl
Fig. 4.43
and
K
when
T
=573 K (
=0.78;
K
=0.002217;
r
2
=0.859)
-1.6
-0.8
-1.8
-1.2
-2.0
-2.2
-1.6
-2.4
-2.6
-2.0
-2.8
2.8
3.2
3.6
4.0
4.4
4.8
2.8
3.2
3.6
4.0
4.4
4.8
ln
C
HCl
ln
C
HCl
Fig. 4.46
and
K
when
T
=1173 K (
=0.80;
K
=0.010584;
r
2
=0.959)
Fig. 4.45
and
K
when
T
=973 K (
=0.79;
K
=0.005312;
r
2
=0.896)
Energy activation and pre-exponential factor
The reaction rate
K
of mercury and HCl at various reaction temperatures has been
obtained as shown in Figs. 4.42 to 4.46. The results are summarized and shown in
Table 4.9. With the use of
K
at different temperatures, the energy activation and
pre-exponential factor of the chemical reaction were calculated to derive the spe-
cific reaction rate equation.
Table 4.9
Reaction rate
K
of mercury and HCl at various reaction temperatures
K
ln
K
T
(K)
1/
T
0.000499
7.6029
373
0.002680965
0.002217
6.1116
573
0.001745201
0.003198
5.74523
773
0.001293661
0.005312
973
0.001027749
5.23779
0.010584
4.54841
1173
0.000852515
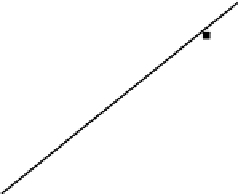
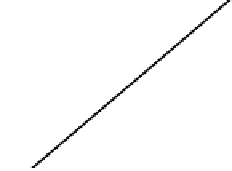
Based on the kinetic model, a straight line could be drawn from the logarithmic
of the reaction rate constant
K
to 1/
T
.
E
a
could be obtained from the slope, and
A
could be obtained from the linear intercept. The results are shown in Fig. 4.47.
























































































Search WWH ::

Custom Search