Environmental Engineering Reference
In-Depth Information
Transformation in Flue Gas
From the simulation results shown in Figs. 4.17 to 4.41, the value of
was
between 1.53 and 1.91, and the average was 1.78. This value is similar to that given
in the literature
[14, 4]
.
2) Reaction order
and reaction rate constant
K
The results shown in Figs. 4.17 to 4.41 determined the reaction rate
K
1
of the
pseudo
-level at a given HCl concentration and reaction temperature, which were
summarized and shown in Table 4.8. The reaction order
of reaction rate to HCl
and the apparent reaction rate constant
K
between mercury and HCl could be ob-
tained from these results.
Table 4.8
Reaction rate
K
1
of the pseudo
-level at a given HCl concentration and reaction tem-
perature
T
(K)
C
HCl
(ppm)
K
1
T
(K)
C
HCl
(ppm)
K
1
373
20
0.00765
773
80
0.07024
373
40
0.0107
773
100
0.1073
373
60
0.01378
973
20
0.0624
373
80
0.03939
973
40
0.09049
373
100
0.03434
973
60
0.1078
573
20
0.0258
973
80
0.1280
573
40
0.02514
973
100
0.1691
573
60
0.07694
1173
20
0.1264
573
80
0.066
1173
40
0.1715
573
100
0.0743
1173
60
0.2290
773
20
0.0364
1173
80
0.4034
773
40
0.03759
1173
100
0.4173
773
60
0.07951
Based on the kinetic model, a straight line could be drawn from the logarithmic
of the pseudo
-level reaction rate constant
K
1
to the logarithmic of HCl concen-
tration.
could be obtained from the slope, and
K
could be obtained from the linear
intercept. The results are shown in Figs. 4.42 to 4.46.
From the simulation results shown in Figs. 4.42 to 4.46, the range of
was
between 0.76 and 0.88, and the average was 0.79. In this way, the specific form of
the kinetics formula of the total combination reaction of mercury and HCl in the
flue gas with other compositions was established.
-3.2
-3.6
-4.0
-4.4
-4.8
2.8
3.2
3.6
4.0
4.4
4.8
ln
C
HCl
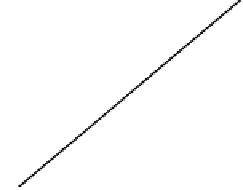
Fig. 4.42
and
K
when
T
˙
373 K (
=0.88;
K
=0.000499;
r
2
=0.861)






























Search WWH ::

Custom Search