Biology Reference
In-Depth Information
(a)
(b)
100
100
80
60
40
20
0
10
1
0.1
0.01
0.001
10
0
50
100
150
200
100
1000
Anacardic acid (
µ
M)
Anacardic acid (
µ
M)
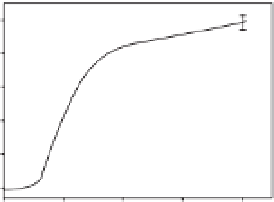
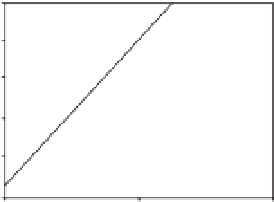
Fig. 9.3.
Inhibited rates of the superoxide anion generation by anacardic acid (C
15:3
) and the Hill plot
analysis. (a) Inhibited rates of superoxide anion generation were calculated from those of superoxide
anion generation by xanthine oxidase in the presence of 0-200
m
M anacardic acid (C
15:3
) at 200
m
M
xanthine. (b) The rates were plotted according to the Hill equation.
very narrow range of anacardic acid (C
15:3
)
concentration (0.04-0.14 mM), which is
much less than a usual simple equilibrium
that would occur over a 100-fold concentra-
tion range. This indicates only tight binding
of inhibitor, but the curve of inhibition rate
followed a Hill equation with a slope factor of
4.2 ± 0.5. This suggests that anacardic acid
(C
15:3
) binds cooperatively to xanthine oxidase
(Bray, 1963). It should be noted, however, that
a common naturally occurring antioxidant,
a-tocopherol, is less effective in scavenging
the superoxide anion generated by the xanthine
oxidase and the IC
50
is 220 ± 20 mM (Masuoka
and Kubo, 2004).
It seems that the antioxidant activity of
anacardic acids is not due to radical scav-
enging but to inhibiting the enzyme activity.
In order to verify this conclusion the forma-
tion of uric acid was measured, because xan-
thine oxidase is known to convert xanthine
to uric acid. This enzyme-catalysed reaction
proceeds via the transfer of an oxygen atom
to xanthine from the molybdenum centre.
The inhibition mechanism also does not fol-
low hyperbolic inhibition by anacardic acid
concentration (Fig. 9.2) but follows the Hill
equation instead. The shape of the inhibi-
tion curve of xanthine oxidase by anacardic
acid (C
15:3
) is sigmoidal (IC
50
= 162 ± 10 mM).
The curve of inhibition rate followed a Hill
equation with a slope factor of 1.7 ± 0.2. This
result confirmed that anacardic acid (C
15:3
)
binds by cooperative binding to xanthine
oxidase and affects the uric acid formation
less than the superoxide anion formation.
Interestingly, salicylic acid did not inhibit
the enzyme up to 200 mM (27.6 mg/ml) but
cooperatively inhibited at higher concentra-
tion (IC
50
= 580 ± 28 mM). The result obtained
indicates that the alkyl side chain plays an
important role in eliciting the activity. The
hydrophobic interaction alone is not
enough, however, to elicit the xanthine oxi-
dase inhibitory activity because cardanol
(C
15:3
), which possesses the same side chain
as anacardic acid (C
15:3
), did not exhibit any
inhibitory activity.
9.6
Lipoxygenase
Lipoxygenase (EC 1.13.11.12) is a non-haem
iron enzyme that catalyses the dioxygena-
tion of polyunsaturated fatty acids contain-
ing a 1(
Z
),4(
Z
)-pentadiene system, such as
linoleic acid and arachidonic acid, into
their 1-hydroperoxy-2(
E
),4(
Z
)-pentadiene
product (Shibata and Axelrod, 1995).
Lipoxygenases are therefore of importance
because they could generate peroxides in
human low-density lipoproteins (LDLs)
in vivo
and facilitate the development of
arteriosclerosis, a process in which lipid
peroxidation seems to be intimately involved
(Cornicelli and Triredi, 1999; Kris-Etherton
and Keen, 2002). Lipid peroxidation is a
typical free-radical oxidation and proceeds
via a cyclic chain reaction (Witting, 1980).














Search WWH ::

Custom Search