Biology Reference
In-Depth Information
It is also well known, however, that lipid
peroxidation is one of the major factors in
deterioration during the storage and process-
ing of foods, because it can lead to the devel-
opment of unpleasant rancid or off flavours
as well as potentially toxic end products. In
our preliminarily assay we became aware
that anacardic acid (C
15:3
) and anacardic
acid (C
15:2
) were oxidized as substrates at
lower concentrations (<40 mM) because both
possess a 1(
Z
),4(
Z
)-pentadiene system in
their C
15
-alkenyl side chain. Hence, the
inhibition kinetics were emphasized with
anacardic acid (C
15:1
), although both ana-
cardic acid (C
15:3
) and anacardic acid (C
15:2
)
inhibited the oxidation of linoleic acid
catalysed by soybean lipoxygenase-1 (EC
1.13.11.12, Type 1) at a higher concentra-
tion (>40 mM).
The oxidation of linoleic acid cata-
lysed by soybean lipoxygenase-1 follows
Michaelis-Menten kinetics. The kinetic
parameters for this oxidase obtained from
a Dixon plot show that the
K
m
value is 11.7
mM and
V
m
is 4.8 mM/min. The estimated
value of
K
m
obtained using a spectrophoto-
metric method is in good agreement with
the previously reported value (Schilstra
et al
., 1992; Berry
et al
., 1997). The kinetic
and inhibition constants obtained are listed
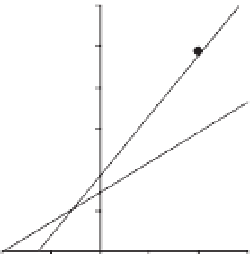
in Table 9.1. As illustrated in Fig. 9.4, the
inhibition kinetics analysed by Dixon
plots show that anacardic acid (C
15:1
) is a
competitive inhibitor because increasing
anacardic acid (C
15:1
) resulted in a family
of lines with a common intercept on the
1/
v
axis but with different slopes. The
equilibrium constant for inhibitor binding,
K
I
, was obtained from a plot of the appar-
ent Michaelis-Menten constant versus the
concentration of anacardic acid (C
15:1
),
which is a linear. The inhibition kinetics
analysed by Lineweaver-Burk plots also
confirmed that the anacardic acid (C
15:1
) is
a competitive inhibitor (data not shown).
A similar result was also obtained by mon-
itoring oxygen consumption and the results
are listed in Table 9.1. The estimated value
Table 9.1.
Kinetics and inhibition constants of
anacardic acid (C
15:1
) for soybean lipoxygenase-1.
Inhibition
Increase of A
234
O
2
consumption
IC
50
6.8
m
M
31.5
m
M
K
m
11.7
m
M
43
m
M
V
m
4.8
m
mol/min
6.5
m
mol/min
Inhibition
Reversible
Reversible
Inhibition type
Competitive
Competitive
K
I
2.8
m
M
14.2
m
M
0.9
(a)
1.2
(b)
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
1.0
0.8
0.6
0.4
0.2
-10
-5
0510
15
-60 -40 -20
020406080
[l] (
µ
M)
[l] (
µ
M)
Fig. 9.4.
Dixon plots of 13-HPOD generation and oxygen consumption by soybean lipoxygenase-1 in the
presence of anacardic acid (C
15:1
) in borate buffer (pH 9.0) at 25°C. (a) Plots of 13-HPOD generation
(increase of A234 nm). Concentrations of linoleic acid substrate for curves
•
and were 15 and 30
m
M,
respectively.
K
m
is equal to 11.7
m
M,
K
I
is equal to 2.8
m
M and
V
m
is equal to 4.8
m
mol/min. (b) Plots of
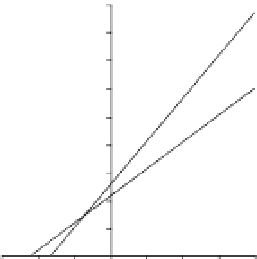
oxygen consumption. Concentrations of linoleic acid for curves were 50 (
•
) and 80 ()
m
M, respectively.
K
m
is equal to 43
m
M,
K
I
is equal to 14.2
m
M and
V
m
is equal to 6.5
m
mol/min.




























Search WWH ::

Custom Search