Environmental Engineering Reference
In-Depth Information
experiments where hydrogen was produced from paper sludge in supercritical water in the
presence of ruthenium (IV) dioxide, RuO
2
. The reaction conditions were 100 mg paper
sludge, 20 mg catalyst, temperature of 450
o
C for 2 hours. The major components of gases
produced were hydrogen, methane and carbon dioxide in molar ratios of 27%, 27% and 45%,
respectively. One of the author's research group has recently started an extensive study on
catalytic gasification of pulp/paper secondary sludge in SCW for hydrogen production. To the
best of the authors' knowledge, to date there is no other investigation worldwide that is
focused on the use of pulp/paper secondary sludge as feedstock for hydrogen generation.
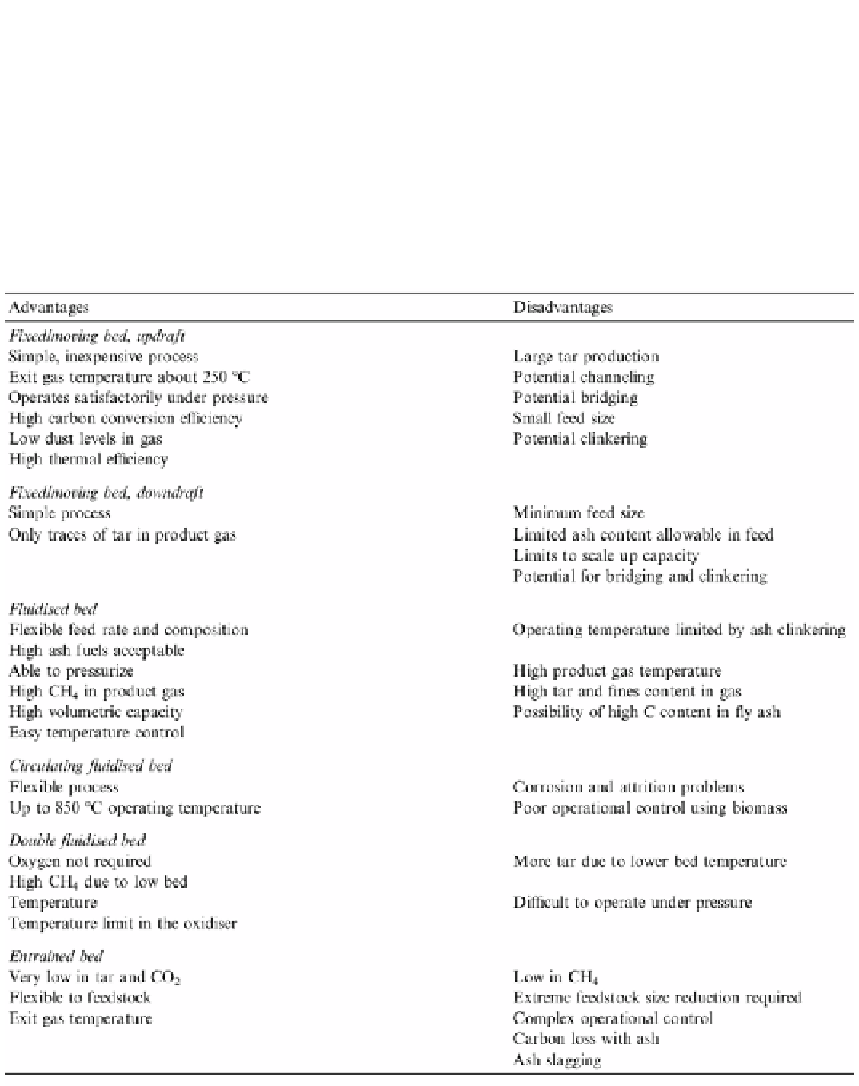
Table 3. Comparison of different types of gasifiers (Rampling and Gill, 1993).
Energy requirement in different stages of a typical gasification process is outlined in
Figure 5, including the location of energy inputs and outputs.
According to Ptasinkski et al. (2007), gasification of raw sludge is not very energy
efficient because the raw sludge contains a substantial amount of water (at as high as 98-99
wt%), and hence the optimal reaction conditions are difficult to achieve. In order to improve
the gasification efficiency, the moisture content of the sludge must be greatly reduced or dried