Chemistry Reference
In-Depth Information
CaO + 3 C
CaC
2
+CO
Calcium carbide
CaC
2
+H
2
O
H
CC
H
+ CaO
Acetylene
Cl
HgCl
2
catalyst
+ HCl
H
CC
H
VCM
Ethylene oxide is an epoxide made by the oxidation of ethylene in the
presence of a catalyst, often a silver catalyst [12]. Ethylene and air are passed
over a catalyst, typically at pressures of 10 - 30 bar and temperatures of
200 - 300
∘
C. The reaction is exothermic and the exotherm must be controlled
to prevent runaway reaction and further oxidation to carbon dioxide. A typi-
cal reactor consists of large bundles of several thousand tubes that are packed
with catalyst. A coolant surrounds the reactor tubes, removing the reaction
heat and permitting temperature control [13]. Ethylene oxide has many uses
including the production of various ethers, alcohols and ethanolamines,
but the largest use is the production of ethylene glycol. Ethylene oxide is
converted to ethylene glycol by reacting the ethylene oxide with an excess
of water in the presence of an acid catalyst. The acid catalyst is often a solid,
such as polymer-bound sulfonic acids. This enables the acid to be readily
separated from the reaction mixture. Although only one equivalent of water
is required by theory, a large excess, perhaps 10:1 to 20:1 water: ethylene
oxide on a molar basis, is used because of the competing reaction between
unreacted ethylene oxide and ethylene glycol to form diethylene glycol.
O
2
Catalyst
H
H
O
CC
H
H
Ethylene
Ethylene oxide
H
+
OH
H
+
O
H
+
−
HO
Ethylene glycol
OH
O
H
H
O
H
H
H
+
OH
H
+
H
+
−
O
O
O
H
O
H
O
H
Diethylene glycol
O
H
OH
OH
Ethylene glycol




















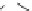


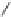
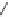



























Search WWH ::

Custom Search