Chemistry Reference
In-Depth Information
Another biological target for many insecticides is the nervous system of
the insect because this can result in selective toxicity. The neonicotinoids, in
group 4, work by this mechanism and act on insect nicotinic acetylcholine
receptors (nAChR). Nicotine, the namesake of the nAChR has been used for
pest control since the 17
th
century [33]. However nicotine is hazardous to
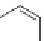
people and has limited effectiveness as an insecticide. Nicotine and its analog
epibatidine, isolated from a tropical poisonous frog, exist mainly in the pro-
tonated form at physiological pH. They are examples of the nicotinoid family
and are toxic to mammals.
Cl
N
N
N
H
H
N
CH
3
Protonated nicotine
H
Protonated epibatidine
In contrast, the neonicotinoids are not protonated and therefore bind differ-
ently, resulting in greater activity on insects than mammals. It is this selective
toxicity that has led to extensive use of the neonicotinoids and they repre-
sent about 20% of the global insecticide market [34]. Worldwide sales of
neonicotinoid insecticides are estimated at $1 billion [35]. Imidacloprid and
thiacloprid are examples of neonicotinoid insecticides.
Cl
Cl
NH
S
N
N
N
N
N
N
NO
2
CN
Imidacloprid
Thiacloprid
Another neonicotinoid example is clothianidin, which is used in the treat-
ment of seeds. Clothianidin is used for canola, cereals, sunflowers, sugar beet,
and corn, including 90% of the United States corn crop [36].
N
Cl
N
S
N
NO
2
HN
CH
3
Clothianidin
There is some concern that neonicotinoids may interfere with the growth
and viability of beehives [37]. Because bees pollinate, thereby playing a key










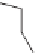






























Search WWH ::

Custom Search