Chemistry Reference
In-Depth Information
blood, and because of the low mammalian toxicity of the pyrethroids.
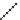
The pyrethroids are characterized as having a cyclopropane carboxylic
acid ester functionality. Some commercial pyrethroids that are used in
mosquito-control
devices
include
d-allethrin,
furamethrin,
prallethrin,
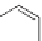
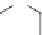
transfluthrin, and metofluthrin [31].
O
O
O
O
O
O
d-allethrin
Furamethrin
F
O
Cl
F
O
O
Cl
O
O
F
F
Prallethrin
Transfluthrin
F
O
CH
3
F
O
O
H
3
C
F
F
Metofluthrin
By synthesizing hundreds of structural analogs and with several struc-
ture activity relationship (SAR) studies, there has been an expansion in the
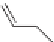
number of synthetic pyrethroids on the market. Type I pyrethroids, repre-
sented by, for example, permethrin cause hyperactivity and incoordination
in the insect [32]. Type II pyrethroids do not have the cyclopropane ring and
contain an alpha-cyanobenzyl ester. Fenvalerate is an example. The Type II
pyrethroids induce paralysis. Note that fenvalerate has two asymmetric car-
bons at the two benzyl positions so there are four possible stereoisomers. It
is sold as a mixture of the four stereoisomers and also as the most active, SS
stereoisomer, which is called esfenvalerate.
O
O
CN
Cl
O
O
O
O
Cl
Cl
Permethrin
Fenvalerate



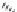































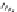











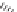




























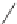




























Search WWH ::

Custom Search