Chemistry Reference
In-Depth Information
also can increase urinary potassium ion loss. Sometimes they are coadmin-
istered with other drugs to compensate for potassium loss. One example is
irbesartan-hydrochlorothiazide (Avalide
®
, Bristol-Myers Squibb). Coad-
ministration of the angiotensin II receptor antagonist tends to reverse the
potassium loss due to the hydrochlorothiazide. It is thought that a decrease in
angiotensin II leads to decreased aldosterone secretion which may cause an
increase in potassium. The same principle is at work when an ACE inhibitor
is
used
with
hydrochlorothiazide
as
in
lisinopril-hydrochlorothiazide
(Prinzide
®
, Merck).
O
O
O
O
S
S
H
2
N
NH
Cl
H
Hydrochlorothiazide
11.4 PROTON PUMP INHIBITORS
Proton pump inhibitors (PPI) are used for people with ulcers and for treatment
of heartburn and other symptoms of gastroesophageal reflux disease (GERD),
a condition in which backward flow of acid from the stomach causes heartburn
and possible injury of the esophagus. GERD has an estimated prevalence of
10 - 20% in the Western world [32] and proton pump inhibitors are the main-
stay of GERD therapy. One study [33] estimated that GERD was prevalent
in 7 million insured people in the U.S. at an incremental health cost of $23
billion. Using a 4% work productivity loss, the cost estimate increases to $32
billion.
The stimulation of the proton pump (H
+
/K
+
-ATPase) in the gastric pari-
etal cell is the final step of acid secretion and proton pump inhibitors block
this enzyme [34]. Omeprazole was launched in 1988 as Losec in Europe
and then in 1990 as Prilosec in the United States [35]. This drug was fol-
lowed by lansoprazole, pantoprazole, rabeprazole and esomeprazole, the S
enantiomer of omeprazole. PPIs are prodrugs that accumulate in the acidic
secretory canaliculi of the gastric parietal calls where they are converted to
the sulfenamide derivative which in turn forms a disulfide bond with the thiol
group of cysteines in the H
+
/K
+
-ATPase causing an irreversible inhibition
of the proton pump. This is shown with omeprazole [36]. Note that due to
the methoxy group on the benzimidazole two isomers of the sulfenamide
are formed. Compared with omeprazole, lansoprazole and rabeprazole have
different substituents on the pyridine ring and pantoprazole has different sub-
stituents on both the pyridine ring and the benzimidazole. The different groups
on both the pyridine ring and the benzimidazole influence the pKa of the pro-
drug and therefore the rate of the conversion to the active metabolite.




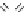


Search WWH ::

Custom Search