Chemistry Reference
In-Depth Information
metals such as barium, cadmium, calcium, and zinc, often in the form of fatty
acid salts such as zinc stearate.
Tin stabilizers are commonly used. There are many types in use but
generally they are monoalkyl or dialkyl tin esters or mercaptides. Common
alkyl groups are methyl, butyl, and octyl. Alkyl tin thioglycolates are
effective heat stabilizers and are commonly used.
OR
′
R
OR
′
R
Sn
S
Sn
S
O
R
O
3
2
Alkyltin thioglycolate
Dialkyltin thioglycolate
9.9 PLASTICIZERS
Plasticizers are compounds added to polymers to facilitate processing
and to increase the flexibility and toughness of the final product. The
plasticizer reduces the intermolecular attractions between polymer chains.
The overwhelming majority of applications impart flexibility to PVC. The
most common plasticizers are aliphatic esters of various carboxylic acids or
aromatic esters of phosphoric acid.
Examples of common plasticizers include phthalate esters such as dioctyl
phthalate (DOP), more properly called di-2-ethylhexyl phthalate (DEHP),
and used as an inexpensive general purpose plasticizer. This is made by react-
ing phthalic anhydride with 2-ethylhexanol. Another example is diisodecyl
phthalate (DIDP). DIDP has lower volatility and improved resistance to
soapy water extraction. It has many applications in PVC used for wire and
cable coating. Phthalate plasticizers are also made from linear alcohols such
as 1-hydroxyheptane, 1-hydroxynonane, and 1-hydroxyundecane. They are
often used when superior low temperature properties, lower volatility, or
outdoor weathering is required.
O
O
O
O
O
O
O
O
DIDP
DOP
Over long periods of time or at higher temperatures the plasticizer
can volatilize from the plastic. Upon aging, PVC can lose its flexibility
and embrittle because of the fugitive nature of plasticizers. Compared
with phthalates, plasticizers based upon trimellitic anhydride offer greater










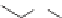


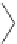


















Search WWH ::

Custom Search