Chemistry Reference
In-Depth Information
9.3 UV STABILIZERS
Many organic compounds, and therefore many polymers, are susceptible to
reactions that are promoted by exposure to ultraviolet light. In a polymer,
this can lead to color change and embrittlement. If a polymer absorbs UV
radiation, the absorbed energy can cause homolytic bond cleavage resulting
in radicals. As in the discussion above about oxidation, the alkyl radicals can
react with oxygen to form peroxy radicals and the peroxy radicals can abstract
a hydrogen atom from a polymer chain to form a hydroperoxide and another
alkyl radical. Especially for articles that have outdoor exposure, some form of
UV stabilizer is often needed. One type of UV stabilizer operates by absorb-
ing the UV energy and then dissipating it by tautomerization. Examples of
this type of stabilizer include the hydroxybenzophenones and the hydroxy-
benzotriazoles.
H
OH
O
O
O
Tautomeriza
t
ion
OC
8
H
17
OC
8
H
17
Typical hydroxybenzophenone stabilizer
H
H
O
O
N
N
N
N
N
Cl
N
Cl
Typical benzotriazole stabilizer
This harmless dissipation of the UV energy stabilizes the polymer. Another
type of UV stabilizer is a class known as hindered amine light stabilizers
(HALS). Many of them are based upon tetramethylpiperidines.
H
Tetramethylpiperidine
Some specific examples are shown with their original Ciba-Geigy Corp.
names, but they are sold under other names by other companies.










































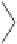
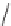

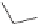




























Search WWH ::

Custom Search