Chemistry Reference
In-Depth Information
CHCH
3
Ethylidene norbornene
There are numerous polyolefin blends. For example, blending a high
molecular weight HDPE with LLDPE gives a blend useful for making thick
films with high modulus (stiffness) and good tear strength [9]. Polypropylene
blended with a maleic anhydride graft ethylene copolymer has improved
adhesion to metals [10]. Ethylene ethyl acrylate (EEA) and ethylene vinyl
acetate (EVA) have been added to polyolefins. They can improve properties
such as surface adhesion for printability. When added to LLDPE, the
toughness and elasticity of the LLDPE is improved [11].
9.2 ANTIOXIDANTS
Whether the plastic article is fabricated from a single polymer or a blend of
polymers, it is subject to oxidative degradation. The susceptibility to oxi-
dation will vary based upon the polymer and the other components in the
formulation. For example, polypropylene which can form tertiary radicals is
more susceptible than HDPE. Some additives can act as catalysts and accel-
erate degradation. The oxidation is a radical reaction. The initial steps are
illustrated with polyethylene, but other polymers have a similar mechanism.
R
H
H
H
H
H
CC
CC
RH +
H
H
x
x
Alkyl radical
OO
O
H
O
H
H
CC
CC
H
H
x
H
x
Peroxy radical
H
O
O
O
H
O
H
H
H
H
H
H
+
CC
+
CC
CC
CC
H
H
H
H
H
H
x
x
x
x
Alkyl hydrogen peroxide
This is only the beginning and both alkenes and carbonyl-containing com-
pounds are eventually formed. Severe oxidation of polyethylene, as might be

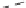






































































Search WWH ::

Custom Search