Chemistry Reference
In-Depth Information
NCO
O
C
N
OCN
N
C
O
NCO
NDI
MDI
HDI
NCO
When a diisocyanate trimerizes, one isocyanate from each of the monomers
forms the trimeric ring and the other remains available for polymerization.
The trimer effectively contains three isocyanates and therefore allows the
polymer to grow in three directions. This is illustrated below with the trimer
of HDI.
NCO
O
C
N
O
O
N
N
C
NN
O
HDI
NCO
O
Trimer
OCN
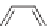
Depending on the desired properties, the diol can be a short chain diol
such as ethylene glycol, 1,4-butanediol, or 1,6-hexanediol or a hydroxyl ter-
minated oligomer such as a polyether diol or a polyester diol. When a polyol
is used, similarly it can be a short chain polyol such as glycerol or it can be
an oligomeric polyol such as polyether polyols or polyester polyols.
Both the diisocyanate portion and the diol/polyol portion are liquids. One
common way to fabricate polyurethanes is by use of reaction injection mold-
ing (RIM). With this method, each of the two liquid components is passed
through a mixer and into a mold where the reaction takes place to form the
polyurethane.
Most polyurethane applications are based upon foamed polyurethanes.
Examples include mattresses, seat cushions, and insulation. Although in
principle any volatile material that can create bubbles can be used to foam a
polymer, carbon dioxide is commonly used because it can be easily generated
by the reaction of the isocyanate linkage with water. The byproduct is an
amine which can react with an isocyanate to form a urea, thereby continuing
the polymer growth. The chemistry is illustrated below.
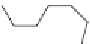



























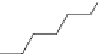




Search WWH ::

Custom Search