Chemistry Reference
In-Depth Information
be made by treating an aromatic diamine with an aromatic diacid chloride
[23, 24].
O
O
O
O
N
H
2
N
NH
2
HN
+
Cl
Cl
x
Kevlar
NH
2
NH
O
O
N
H
2
N
+
Cl
Cl
O
x
O
Nomex
Both can be spun into fibers. Kevlar is used in bullet resistant vests and
Nomex is used in flame retardant protective clothing.
8.8 POLYIMIDE
An imide is made by reaction of an anhydride with an amine. In the first
step, an amic acid is made. Upon heating, water is evolved and the amic acid
closes to the imide. The reaction sequence is illustrated below with phthalic
anhydride and aniline.
O
O
O
H
OH
Heat
+
H
2
N
+H
2
O
N
O
O
O
O
Amic acid
Imide
Polyimides are made similarly from diamines and dianhydrides. Dupont's
Kapton
®
polyimide was the first commercially significant polyimide. It is
made from pyromellitic dianhydride and oxydianiline. Kapton has good heat
and strength properties and is used for specialty applications including space
applications. It is used in electronics and in both film and tape applications.
O
O
O
O
+
H
2
N
O
O
O
N
N
O
O
O
O
NH
2
O
Pyromellitic dianhydride
Oxydianiline
x
Kapton
Another commercial polyimide, Ultem
®
was invented by GE scientists
[25]. It is made from a derivative of BPA dianhydride and m-phenylene





































































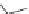
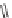


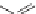
























































Search WWH ::

Custom Search