Chemistry Reference
In-Depth Information
Now, let's consider the same polymer with the stereochemistry inverted at
carbon b. The squiggly lines in the repeating structures mean that the direction
is not assigned.
a
b
H
H
H
H
x
y
Now, the phenyl groups on carbons a and b are on the opposite side of
the polymer chain. This is a different structure and one cannot be converted
to the other without bond breaking. Because of this, polystyrene can have
different structures depending on which side of the ring the phenyl group
is located. Now consider the stereochemistry along an entire chain. When
a polymer structure is written, typically only one unit is written with
parentheses or brackets indicating that the unit repeats. However, to illustrate
the stereochemistry, several units are depicted. In this structure, all of the
groups attached along the polymer chain are on the same side of the polymer.
The stereochemistry of a polymer is called the tacticity and a polymer with
all groups on the same side is called isotactic.
H
H
H
H
H
H
H
H
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
x
Isotactic polystyrene
If the groups are randomly arranged, the polymer is said to be atactic. When
the groups alternate from side to side, the polymer is syndiotactic.
Ph
H
H
H
H
H
H
H
H
Ph
Ph
Ph
Ph
Ph
Ph
Ph
x
Atactic polystyrene
H
H
H
H
H
H
H
H
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
x
Syndiotactic polystyrene
The olefin polymers such as polystyrene, polyethylene, and polypropylene
are all addition polymers. In an addition polymer, the monomer units are
“added” together with each of the atoms of the monomer incorporated into the


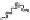
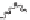

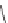


















































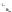


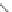
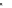













Search WWH ::

Custom Search