Chemistry Reference
In-Depth Information
Polystyrene can also be made by using organometallic catalysts to
polymerize styrene. Polymers can have stereochemistry and the use of
certain organometallic catalysts is a means to control stereochemistry.
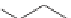
Consider two conformers of 1,3-diphenylpropane.
H
H
H
H
H
H
H
H
Because there is free rotation of the single bonds, these conformers
rapidly interconvert and there is only one 1,3-diphenylpropane. These two
conformers are not different compounds. Just as a person walking down the
street remains the same person and we don't have one person with the left
foot forward and another person with the right foot forward, the conformers
are different representations of the same molecule.
Now consider 2,4-diphenylhexane. The two benzylic carbons (the carbons
directly attached to the phenyl groups) each have four different groups
attached to them, and therefore they are each chiral carbons. There is no
plane of symmetry in the molecule and therefore there are different stereoiso-
mers. The two stereoisomers depicted below (they are diastereomers) cannot
be interconverted by bond rotation.
H
H
H
H
H
3
C
CH
2
CH
3
H
3
C
CH
2
CH
3
In the case of a polymer, we can have a similar situation. Consider the
benzylic carbons on two neighboring units within a polystyrene polymer
chain, arbitrarily designated as carbon “a” and carbon “b” in the structure.
These carbons are asymmetric or chiral. In this structure, the phenyl groups
on carbons a and b are on the same side of the polymer chain.
a
b
H
H
H
H
x
y




















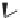
























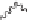












































Search WWH ::

Custom Search