Geoscience Reference
In-Depth Information
Methane solubility in sodium chloride brine
The solubility of methane in brine is estimated using the modified
Spivey et al. correlation for methane solubility in brine as follows.
Step 8.
First, calculate the vapor pressure of pure water,
p
σ
, from the
IAWPS-95 formulation, given in equation (4.13).
23
,
(4.13)
where
T
is K,
T
c
= 647.096, K,
p
c
= 22.064 MPa, and
ϑ
is defined as
(4.14)
The coefficients for equation (4.13) are given in table 4-9.
Table 4-9. Coefficients for international standard water vapor-pressure equation, equation (4.13)
Coeficient
Value
Coeficient
Value
a
1
-7.85951783
a
4
22.6807411
a
2
1.84408259
a
5
-15.9618719
a
3
-11.7866497
a
6
1.80122502
Step 9.
Calculate the solubility of methane in pure water at the
temperature and pressure of interest.
First, calculate the methane solubility coefficients
A
(
T
),
B
(
T
), and
C
(
T
) using equation (4.1), with the coefficients given in table 4-10.
Table 4-10. Coefficients for the modified Spivey et al. correlation for methane solubility in pure
water for use in equation (4.1)
a
i
A
(
T
)
B
(
T
)
C
(
T
)
a
1
0.0
-0.03602
0.6855
a
2
-0.004462
0.18917
-3.1992
a
3
-0.06763
0.97242
-3.7968
a
4
0
0
0.07711
a
5
0
0
0.2229








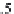








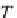



















































































Search WWH ::

Custom Search