Geoscience Reference
In-Depth Information
Next, calculate the solubility of methane in pure water from equation
(4.15).
(4.15)
Step 10.
Calculate the solubility of methane in brine at the temperature
and pressure of interest, as follows.
Calculate coefficients
λ
CH
4
,Na
and
ζ
CH
4
,NaCl
from equations (4.16) and
(4.17), respectively, using the appropriate coefficients from table 4-11.
The functional form for the coefficients
λ
CH
4
,Na
and
ζ
CH
4
,NaCl
is similar
to that used by Duan-Mao, but pressure is in MPa, and the pressure-
squared term in equation (4.16) is independent of temperature.
24
Note that temperature in equation (4.16) is Kelvin, and pressure
is MPa.
(4.16)
(4.17)
Table 4-11. Coefficients for the modified Spivey et al. correlation for methane solubility in brine
μ
l
C
(
o
4
)
λ
CH
4
,Na
, Eq. (4.16)
ζ
CH
4
,NaCl
, Eq. (4.17)
, Eq. (4.19)
Coefficient
RT
c
1
8.3143711
-0.80898
-3.89E-03
c
2
-7.2772168E-04
1.0827E-03
c
3
2.1489858E+03
183.85
-1.4019672E-05
c
4
c
5
-6.6743449E+05
c
6
7.6985890E-02
3.924E-04
c
7
-5.0253331E-05
c
8
-30.092013
c
9
4.8468502E+03
c
10
0
-1.97E-06
Next, calculate the methane solubility in brine, in g-mol/kg H
2
O,
from equation (4.18).
m
CH
4
,b
=
m
CH
4
,w
EXP[-2
λ
(
T
,
p
)
m
Na
-
ζ
(
T
,
p
)
m
NaCl
2
]
(4.18)












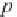



























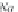
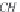





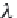








































































Search WWH ::

Custom Search