Biomedical Engineering Reference
In-Depth Information
This equation can be integrated as an exponential law, and using (7.21), we
ind
d P
[
]
k c
2 1
=
exp( (
-
k
+
k
) )
t
2
-
1
d t
(
k
+
k
)
2
-
1
where
c
1
is a constant. Integrating once more, we obtain the form
k c
2 1
[
P
]
= -
exp( (
-
k
+
k
) )
t
+
c
2
-
1
2
(
k
+
k
)
2
-
1
Using the value of the asymptote [
P
]
¥
and continuity at time
t
1
, the “asymp-
totic” regime is defined by
V
[ ]
S t
max
0 0
(7.34)
[
P
]
=
[
P
]
-
exp [
-
k K t
(
-
t
)]
1
m
1
¥
K
m
So, the three different regimes and their assumptions have been identified: para-
bolic at first when [
ES
] is small, then linear when
d
[
ES
]
/dt
= 0, finally asymptotic
when [
S
] becomes small. The system of (7.32), (7.33), and (7.34) is more accurate
that the Michaelis-Menten law, but requires the knowledge of four parameters in-
stead of 2:
V
max
,
K
m
,
k
2
and [
P
]
¥
(it can be shown that the times
t
0
and
t
1
may be
deduced from considerations on the derivability of the kinetic curve). In the follow-
ing section, we show on an example the difference between the two models.
7.3.2.3 Example of Enzymatic Reaction
In this example, we set up a catalyst reaction between a synthetic protein and an
enzyme. Consider a substrate composed of molecules of BAEE (Benzoyl-Arginyl-
Ethyl-Esther) - which is a synthetic protein—reacting in presence of trypsine—which
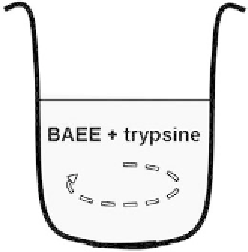
is an enzyme. The experiment consists in mixing the substrate
S
(synthetic protein)
with the enzyme
E
(trypsine) in a small beaker (Figure 7.19).
Reaction kinetics is measured by an optical method based on the absorbance of
light by the reaction product
B
. Figure 7.20 shows the absorbance curves at differ-
ent times for three different initial concentrations of BAEE. Kinetics plots are then
deduced from light absorbance.
Figure 7.19
Mixing BAEE and trypsine.