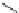Geoscience Reference
In-Depth Information
Although the carbon system has been investigated for the past several decades by
researchers worldwide, most of the solid forms of carbon-like diamond such as carbon
(amorphous carbon), carbon nanocells, fullerenes, and graphene still do not find a
place in the carbon phase diagram, which gives only the stable fields of phases like
graphite, diamond, and liquid phase. Graphite and diamond occur in contrasting
geochemical environments. The exact processes that control their formation in nature
are still debatable. But it is very well known that the P
Hfluid
systems have played a significant role in the formation of these two pure forms.
Here, we shall consider only the wet chemical routes, particularly hydrothermal,
solvothermal, and supercritical fluid techniques of synthesis of nanoforms of carbon.
Ever since De Vries
[64]
first suggested the possibilities of hydrothermal synthesis
of diamonds based on some of the geological evidences like syngenetic C
T
fO
2
and C
O
O
fluid and silicate mineral inclusions in natural diamonds, several researchers
attempted the synthesis of nanosize and bigger size diamond crystals and other
carbon-based nanomaterials using the hydrothermal method [70]. Different carbon
sources were attempted with varying success rate. The hydrothermal and solvother-
mal techniques are highly promising for reactions involving volatiles as they attain
the supercritical fluid state, and supercritical fluids are known for their greater abil-
ity to dissolve nonvolatile solids. Silicon carbide powder has been used for the
synthesis of carbon polymorphs, and Gogotsi et al.
[72]
have reported decomposition
of silicon carbide in supercritical water and discussed the formation of various car-
bon polymorphs. In 1998, Li et al. (1998)
[73]
succeeded in synthesizing diamond
particles through a metallic reduction
H
catalysis route based on the
Wurtz-like reaction of carbon tetrachloride and sodium at 700
C. This inspired the
synthesis of CNTs using a carbon precursor like hexachlorobenzene, with a planar
hexagon configuration
[74]
. The scheme below illustrates the catalytic assembly
benzene-thermal route to multiwalled CNTs with an average diameter of 40 nm at a
moderate temperature. Co/Ni catalyst was used at a temperature of 350
C. This is
perhaps a breakthrough in the reduction of experimental temperature of synthesis of
CNTs, though yield of CNTs was relatively low (
pyrolysis
10%).
B
Cl
Cl
Cl
350° C
Benzene
n
+ 6nK
6nKCl+
Cl
Cl
Cl
350° C
Co/Ni
Carbon nanotube
The catalytic assembly benzene-thermal route to multiwalled CNTs with an average diameter
of 40 nm at a moderate temperature. Co/Ni catalyst was used at a temperature of 350
C
[74]
.
Later, several researchers explored the possibility of solvothermal synthesis of car-
bon nanoforms. Basavalingu et al. (2001) have synthesized carbon polymorphs under
solvothermal conditions through decomposition of silicon carbide in the presence of