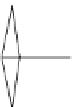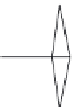Geoscience Reference
In-Depth Information
Figure 6.24 Sodalite cage containing M
4
X cluster
[124]
.
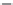
Figure 6.25 Dependence of solubility of
sodalite and the NaOH concentration at
constant temperature
[123]
.
S
(wt%)
3
2
1
0
10
20
30
C
NaOH
(wt%)
40
Mel'nikov et al.
[122]
have carried out the hydrothermal growth of bulk sodalite
crystals using seed crystals and have studied the kinetics of crystallization in detail.
The experimental temperature was varied from 150
Cto400
C in steps of 50
C.
Figure 6.26
shows the relationship between the growth rates of sodalite crystals in the
directions and the temperature gradients: (a) 200
C, (b) 250
C, (c) 300
C, and (d)
350
C. The mass transfer associated with the crystallization of sodalite depends line-
arly on the temperature gradient for a constant growth temperature. They have found a
direct relationship between the growth rates of the sodalite faces and the temperature
drop, at constant temperature, and an exponential temperature dependence at constant
supersaturation
[122]
. Recently, Hayashi et al.
[128]
have grown millimeter-sized alu-
minosilicate sodalite single crystals under hydrothermal conditions.
In spite of the interesting results obtained by the above authors, the quantum of
data available on crystal growth is just meager compared to the data on the synthe-
sis of zeolites. It has to be understood in greater detail.
6.7 Aluminophosphate Zeolites
Aluminophosphate zeolites represent a new class of microporous inorganic solids
with the potential
to be as useful and as scientifically challenging as the