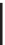Geoscience Reference
In-Depth Information
Weak crystal field
e
g
Δ
t
2
g
High-spin Fe
2+
Strong crystal field
e
g
Δ
t
2
g
Low-spin Fe
2+
Figure 1.9
Two electronic configurations for the same ion Fe
2
+
with 6d electrons. When the crystal field is
strong, the energy gap
between the
t
2
g
and
e
g
orbitals increases, and the six electrons fill the
three
t
2
g
orbitals. This is the low-spin configuration. When the crystal field is weak, the energy
cost of pairing electrons dominates and two electrons move to the upper
e
g
orbitals. This is the
low-spin configuration. Only some ions of transition elements show such a dual configuration.
field is 4
×
(
−
2
/
5
)
+
2
×
(
3
/
5
)
=−
2
/
5. Conversely, the large
case (full
t
2
g
)
5.
3. For divalent nickel Ni
2
+
([Ar]3d
8
4s
0
), the configuration is unique: the three
t
2
g
orbitals
are fully occupied, while each of the
e
g
orbitals hosts one electron. The energy gain
is 6
is referred to as “low-spin” Fe and has a crystal field effect of
−
12
/
5; Ni
2
+
therefore snuggles in octahedral sites
and is notably enriched in Fe-Mg silicates such as olivine and pyroxenes.
4. The common high-spin Mn
2
+
([Ar]3d
5
4s
0
)aswellasZn
2
+
([Ar]3d
10
4s
0
)havesym-
metrical orbital configurations and therefore no energy gain: in general, they show little
preference between silicate minerals or between silicates and melts.
×
(
−
2
/
5
)
+
2
×
(
3
/
5
)
=−
6
/
The effect of the crystal field is essentially symmetrical when these ions occur in tetrahedral
coordination.
In solutions, transition-element compounds form by interaction between a ligand and
a cation, which is the basis of Lewis acid-base theory. Typical of such interaction is
hydration, e.g. for Zn
Zn
2
+
(OH)
−
+
Zn
2
+
+
H
+
H
2
O
⇔
(1.3)