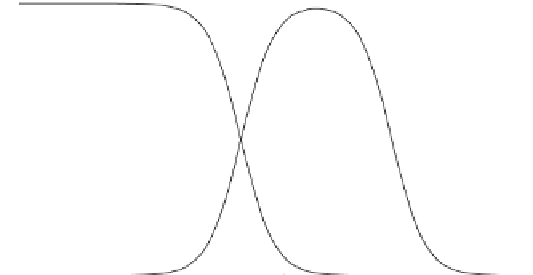Geoscience Reference
In-Depth Information
7.7 The carbonate system
By using methods from solution chemistry, it is possible to calculate the speciation of
carbonates in water, for example the
α
HCO
3
fraction of carbonate dissolved as bicarbonate
ions. This parameter is defined as:
HCO
3
α
HCO
3
=
CO
2
3
(7.33)
+
HCO
3
+
[H
2
CO
3
]
K
1
H
+
α
HCO
3
=
(7.34)
K
1
H
+
+
H
+
2
K
1
K
2
+
with similar expressions for
α
H
2
CO
3
. Carbonate speciation therefore only
depends on [H
+
]. If these expressions are plotted against pH, three dominance zones can
low pH values, the surplus of protons leaves all the carbonates as carbonic acid H
2
CO
3
,
while at high pH the deficit of protons favors CO
2
3
;HCO
3
is the dominant ion in the
intermediate pH zone. Natural water is weakly acidic or weakly basic. Seawater, with a pH
of 7.6-8.0, is largely dominated by HCO
3
ions.
The carbonate system in a solution in contact with the atmosphere is controlled by the
chemical variables
P
CO
2
, pH, alkalinity
Alk
α
CO
2
3
and
2
CO
2
3
, the sum of available
≈
HCO
3
+
CO
2
3
(omitting H
2
CO
3
and atmospheric CO
2
), and
CO
2
≈
HCO
3
+
carbonates
Acidic
Basic
1.0
CO
2
−
H
2
CO
3
HCO
−
0.8
0.6
0.4
0.2
p
K
1
p
K
2
0.0
0
2
4
6
8
10
12
14
pH
Figure 7.5
Speciation of dissolved carbonates as a function of the pH of the solution (see
(7.34
)
). Notice that
the predominance of each carbonate species changes when pH values equal to p
K
are crossed.
Much natural water has a pH close to neutral and is therefore dominated by the bicarbonate
ion HCO
3
.