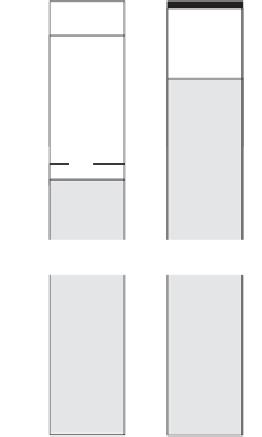
Geoscience Reference
In-Depth Information
Alkalinity =
650
−
2
−
HCO
3
+
2CO
3
600
2Ca
2
+
2SO
2
−
550
2Mg
2
+
500
K
+
450
100
Na
+
Cl
−
50
0
Cations
Anions
Figure 7.6
The composition of seawater in number of charges (equivalent) per unit weight. Alkalinity (2 meq
kg
−
1
in seawater) is the deficit of charge carried by totally dissociated (therefore chemically
inert) anions compared with the charge carried by totally dissociated cations. In a solution, this
deficit, which is essentially balanced by carbonates, is constant. It is a measure of its capacity to
neutralize strong acids (after Broecker,
1994
).
the physical variables of temperature and, to a lesser extent, pressure. These variables are
not independent and we seek to work with conservative variables, which exclude pH and
carbonate concentrations. Combining the expressions for
Alk
and
CO
2
gives:
CO
2
3
=
Alk
−
CO
2
HCO
3
=
2
CO
2
−
Alk
(7.35)
a number of charges expressed in molar units). Some 90% of dissolved carbonate is in
the form [HCO
3
]. Alkalinity is much lower, or even negative, in rain water and river
water. By combining these expressions with those describing the solubility of CO
2
and
the dissociation of carbonic acid, we obtain the very important equation:
2
k
CO
2
K
1
(
K
2
2
CO
2
−
Alk
)
P
CO
2
=
(7.36)
(
Alk
−
CO
2
)
pressures are expressed in ppmv (parts per million volume) and it is taken that at 25
◦
C,
k
CO
2
is approximately equal to 0.029 moles per kg and per atm. The pH of seawater can