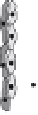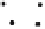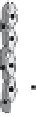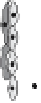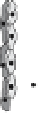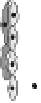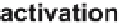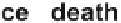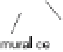Biomedical Engineering Reference
In-Depth Information
This article reviews the design and potential importance of polymeric ve-
hicles for bioinspired supply of angiogenic growth factors, and VEGF will be
used as a paradigm for the discussion of specific design parameters for bioin-
spired growth factor delivery. A short overview of VEGF's biology precedes
the description of design variables. Currently, therapeutic angiogenesis, bone
regeneration, and nerve regeneration represent the best understood areas for
VEGF delivery from polymeric systems, and multifactor approaches for these
specific applications will be described.
2
VEGF Biology
Angiogenesis is a complex multilevel process regulated by a well-concerted
interplay between numerous cell types, proteolytic enzymes, cytokines, and
growth factors, and VEGF is one of the most widely studied angiogenic fac-
tors [16-18]. It initiates activation, migration, and proliferation of endothelial
cells to sprout neovessels (Fig. 2). These newly formed tubes are stabilized
through recruitment of and association with mural cells (smooth muscle cells
and pericytes) [18-21]. Withdrawal of VEGF prior to stabilization causes re-
gression of nascent vessels due to endothelial cell death. During this sequence
of events, VEGF acts in cooperation with other growth factors [18-21]. While
fibroblast growth factor (bFGF) and angiopoietin-2 (Ang-2) collaborate in the
initiation of the cascade, platelet derived growth factor (PDGF), transform-
ing growth factor-beta (TGF-beta), and angiopoietin-1 (Ang-1) are required
in later stages mediating maturation of neovessels by promoting interactions
Fig. 2
Angiogenesis is a multistep process involving the synergistic interplay of different
growth factors and cell types. VEGF initiates the angiogenic cascade in cooperation with
bFGF and Ang-2. Mature and functional blood vessels develop in the presence of PDGF,
TGF-beta, and Ang-1; VEGF withdrawal before maturation leads to endothelial cell death