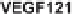Biomedical Engineering Reference
In-Depth Information
with mural cells (Fig. 2) [18-20]. In order to regenerate functional blood ves-
sels, VEGF may need to be constantly supplied over an extended time period,
and in combination with other growth factors.
Polymer systems used for VEGF delivery mimic key aspects by which the
extracellular matrix (ECM) of tissues controls the bioavailability and signal-
ing activity of VEGF in the body. Specific characteristics of the ECM regulate
the versatile functions of VEGF in tissue regeneration, and provide design cri-
teria for polymeric delivery systems. Specifically, the ECM sequesters VEGF
and enables storage of this otherwise rapidly biodegraded factor, presents it
in a localized fashion, and enhances the efficiency of signal transduction. Bio-
logic control over VEGF binding to the ECM, and thus its bioavailability, is re-
alized by cellular production of four different isoforms (VEGF121, VEGF165,
VEGF189, and VEGF206). A process called alternative splicing generates all
four isoforms from the same gene. To what extent the individual isoforms
are bound to the ECM is determined by the molecular size of their ECM-
binding regions (Fig. 3). VEGF121 completely lacks this part of the molecule
and is, therefore, freely diffusible and immediately bioavailable upon secre-
tion from cells. VEGF165, the predominant isoform in the body, exists in
both free and bound forms, whereas VEGF189 and VEGF209 are almost com-
pletely sequestered, and only liberated on cellular demand (Fig. 3) [22-24].
Cells produce proteolytic, ECM-degrading enzymes (e.g., heparanase or ma-
trix metalloproteinases [MMPs]), and these ultimately trigger the release of
soluble, bioactive VEGF from its ECM depots [23, 24]. Furthermore, mechan-
ical stimuli contribute to the release of VEGF from its ECM depots [25].
ECM-binding of VEGF significantly modulates the interactions with its
receptors, and consequently, plays an important role in the physiological con-
trol of VEGF signal transmission. VEGF receptor-2 (VEGFR-2), currently
considered the main receptor, is expressed by a variety of cells, including en-
dothelial cells and nerve cells [14, 22] and transmits signals in cooperation
with neuropilin-1 (NRP-1). ECM-binding improves the interactions between
Fig. 3
VEGF exists in four different isoforms that are generated from a single gene and
exhibit distinct ECM binding characteristics. Lack of ECM binding allows free diffusion
of the VEGF through tissues, and immediate signaling, whereas VEGF sequestered in the
ECM represents a depot form that is bioavailable on cellular demand