Biomedical Engineering Reference
In-Depth Information
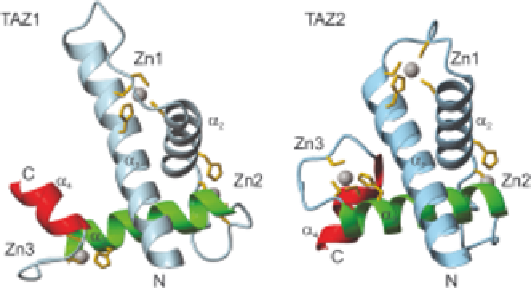
Figure 5.3
Ribbon diagram of the structures of TAZ1 and TAZ2. Each TAZ domain
contains four helices (a
1
-a
4
) and three zinc-binding clusters (Zn1-Zn3).
The a
1
-a
3
helices (blue and green) are structurally homologous, but a
4
(red), is in opposite relative orientation in TAZ1 compared to TAZ2, and
a1 is longer in TAZ1 than in TAZ2. Reproduced with permission from
ref. 89. # Landes Bioscience, 2004.
Within CBP, the TAZ domains play an important role in the recruitment of
CBP to signal-activated transcription complexes, in many cases acting as a
gatekeeper, with competition between various ligands for binding. The NMR
structures of TAZ1 include the free domain
47
and various complexes, with the
transcription factor Hif-1a
48,49
(Figure 5.4), the transcriptional repressor
CITED2
50,51
(Figure 5.5) and the signal transducer and activator protein
STAT2
52
(Figure 5.6). The activation domains of these proteins undergo
coupled folding and binding to TAZ1, and bind in overlapping regions of the
central part of the TAZ1 domain, but the binding sites are not the same.
Surprisingly, in some cases, even the direction of the binding (N- to C-terminal)
of the ligands is different, suggesting that the replacement of one domain by
another could proceed by 'peeling off' the bound domain by a competing
domain that is initially bound at an independent site on the same molecule.
5.3.1.3 The Unstructured NCBD of CBP
The amino acid sequence of the nuclear coactivator binding domain (NCBD)
is not characteristic of a folded protein, but it does contain some hydrophobic
and charged residues. When free in solution, the NCBD is not completely
disordered. Although it shows the presence of some residual helical structure
by CD spectroscopy, it does not show a cooperative unfolding transition.
33
By
NMR it appears more like a molten globule structure, with secondary
structure present, but with unstable and heterogeneous tertiary structure.
33,53
Complexes of NCBD with various ligands are cooperatively folded and highly
helical,
33,54,55
but show considerable structural diversity both of the ligands
and of the NCBD itself. The interaction of two disordered or marginally
structured partners to form a stable folded and highly cooperative complex has
been termed 'mutual synergistic folding',
33
illustrated in Figure 5.7.