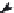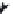Biomedical Engineering Reference
In-Depth Information
Priority 4
H
H
Priority 1
HO
OH
H
3
C
CO
2
H
HO
2
C
CH
3
Priority 3
Priority 2
(a)
H
H
HO
HO
2
C
CH
3
CH
3
CO
2
H
OH
(
S
) Isomer
(
R
) Isomer
(b)
FIGURE 5.2
Procedure for assigning stereogenic centers as possessing either (
R
) or (
S
) coni guration.
(a) Assign priorities according to the CIP rules. (b) View from opposite the group of lowest priority: Clockwise
rotation (13) is (
R
); anticlockwise rotation is (
S
).
i.e., the coni guration of atoms or groups about the stereogenic center. This was rectii ed by the
introduction of the Fischer convention, which labeled such centers as having either d or l con-
i guration based on an arbitrary standard, (+)-glyceraldehyde. However, this system has now been
superseded by the Cahn-Ingold-Prelog (CIP) system that can be used to unambiguously assign any
stereogenic center as possessing either (
R
) or (
S
) stereochemistry. Explanation of the CIP rules can
be found in any general organic chemistry textbook. Once the priorities of the substituents have
been assigned enantiomers are readily classii ed as being the (
R
) or (
S
) isomers. Lactic acid is again
used as an example to demonstrate this (Figure 5.2).
Molecules such as lactic acid are relatively simple in that they only have one stereogenic center.
But what are the implications if multiple stereogenic centers are present? As an example, the drug
ephedrine has two stereogenic centers and thus there are four possible isomers (Figure 5.3). Of
these, the isomers that are mirror images are enantiomers, while the nonsuperimposable nonmirror
images are called diastereomers. It is important to note that diastereomers, unlike enantiomers, will
(unless by coincidence) have nonidentical physical and chemical properties such as boiling point,
solubility, and spectral properties. The potential applications of these differences are discussed in
Sections 5.5.1 and 5.5.2.
As a general rule, the total number of isomers of any given molecule is also given by the rule:
Number of isomers = 2
n
, where
n
is the total number of stereogenic centers
So, as in ephedrine, a compound with two stereogenic centers will have four isomers, three centers
leads to eight isomers, and so on. However, there are exceptions to this rule, because some isomers
may be meso compounds. These can be described as isomers that contain stereogenic centers but
are achiral (and optically inactive) due to the presence of a symmetry plane. Figure 5.4 shows the
example of tartaric acid, with two stereogenic centers and three isomers.
The dei nition of optical purity discussed earlier has been largely superseded by two related
terms: enantiomeric excess (ee, or the proportion of the major enantiomer less that of the minor