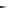Biomedical Engineering Reference
In-Depth Information
CH
3
CH
3
CH
3
HO
H
3
C
OH
H
H
Enantiomers
(mirror images)
(1
S
,2
R
)
(1
R
,2
S
)
Diastereomers
CH
3
CH
3
HO
CH
3
H
3
C
OH
H
H
Enantiomers
(mirror images)
(1
R
,2
R
)
(1
S
,2
S
)
FIGURE 5.3
The relationship between enantiomers and diastereomers. The biologically active forms of
ephedrine are those with the (1
R
, 2
S
)- and (1
S
, 2
S
) coni gurations, which are diastereomers of each other.
CO
2
H
CO
2
H
CO
2
H
CO
2
H
H
OH
HO
H
H
OH
HO
H
Plane of
symmetry
HO
H
H
OH
H
OH
HO
H
CO
2
H
CO
2
H
CO
2
H
CO
2
H
(
R
,
R
)
(
S
,
S
)
(
R
,
S
) (
S
,
R
)
Achiral—meso form
FIGURE 5.4
Tartaric acid has two stereogenic centers but only three stereoisomers.
enantiomer) and diastereomeric excess (de, proportion of the major diastereomer less that of the
minor one). Both ee and de are usually expressed as percentages.
If the only difference between enantiomers was their interaction with plane polarized light, then
their existence would be little more than academic. However, stereochemistry has important impli-
cations in terms of biological activity as described in the following section.
5.3 THE ORIGIN OF STEREOSPECIFICITY IN MOLECULAR RECOGNITION
The lock-and-key hypothesis proposed by Fischer in 1896 was the i rst attempt to explain the
complementarity between a substrate's shape and an enzyme's active site (see also Section 1.1).
Koshland's later hypothesis allowed the enzyme to change its shape to accommodate the binding of
the substrate (induced i t model). Now we know that both the substrate and the enzyme can change
conformation to some extent to ensure optimal binding. Although proposed for enzymes, these
models can also be used as the basis to explain drug-receptor interactions.
Receptors (like enzymes) are made up of amino acids, all of which apart from glycine are chiral.
The interaction of chiral drugs with receptors would be expected to be enantioselective (i.e., one
enantiomer binds with higher afi nity than the other). In order to explain the stereoselective action
of drugs on receptors, the three-point receptor theory was proposed (Figure 5.5). In this theory only