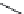Biomedical Engineering Reference
In-Depth Information
model representation produced by CATALYST explicitly includes the physicochemical properties of
the pharmacophore elements and that the model directly can be used for database searching as will
be described in the following text. The physicochemical properties of the predei ned pharmacophore
elements in CATALYST are hydrogen-bond acceptor, hydrogen-bond donor, hydrophobic (aliphatic
or aromatic), negative or positive charge, negatively or positively ionizable, and ring aromatic. In
order to allow for variations in the geometry of the interaction between a ligand and its receptor,
distance variation as well as angle variation is taken into account. For instance, a hydrogen-bond
acceptor is dei ned by a distance from the atom, which accepts a hydrogen bond to the site that
donates the hydrogen bond with an allowed geometrical variation at both ends. These allowed varia-
tions are dei ned by spheres and the optimal interaction is dei ned by the center of the spheres.
The resulting initial CATALYST pharmacophore model with the pharmacophore elements of
the l avone skeleton is shown in Figure 3.2 (bottom right). The carbonyl oxygen atom and the ether
atom are mapped as hydrogen-bond acceptors (green spheres), while the phenyl rings are mapped as
hydrophobic pharmacophore elements (cyan spheres). It should be noted that these are both mapped
as a single sphere whereas the pharmacophore elements involving the carbonyl group and the ether
oxygen are displayed as two spheres in order to take the direction of the modeled hydrogen-bond
interaction into account. For example, the sphere centered at the carbonyl oxygen in Figure 3.2 repre-
sents the ligand side (the hydrogen bond accepting side) of the hydrogen-bond interaction whereas the
outer sphere represents the receptor side (the hydrogen bond donating site) of the hydrogen bond.
3.4.2 R
ECEPTOR
E
SSENTIAL
V
OLUMES
AND
THE
U
SE
OF
E
XCLUSION
S
PHERES
Inactive compounds, which i t the initial pharmacophore are, as mentioned earlier, useful to estab-
lish the dimensions of the receptor binding site, i.e., to determine parts of the binding cavity where
substituents are in steric conl ict with the receptor. Figure 3.3 shows four compounds (
3.6
-
3.9
)
O
O
O
O
HO
O
O
O
O
3.6
K
i
= 4200 nM
3.7
K
i
= >590 nM
3.8
K
i
= 4400 nM
3.9
K
i
= >1500 nM
Exclusion
sphere
FIGURE 3.3
Compounds
3.6
-
3.9
and the pharmacophore model including exclusion spheres (black).