Biomedical Engineering Reference
In-Depth Information
O
O
NO
2
O
O
5
Br
3
2
6
7
4
6'
5'
O
O
O
O
1'
8
1
4'
2'
3'
NO
2
O
O
3.2
K
i
= 70 nM
3.3
K
i
=1.9 nM
3.4
K
i
= 180 nM
3.1
Flavone
Hydrogen-bond acceptor
NO
2
O
Br
Hydrophobic
pharmacophore
element
O
Hydrophobic
pharmacophore
element
O
O
3.5
Template molecule
Hydrogen-bond acceptor
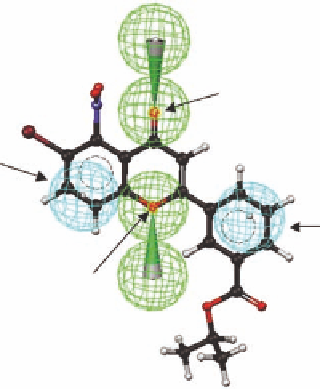
FIGURE 3.2
The structure and atom numbering of l avone (
3.1
), the l avone derivatives (
3.2
-
3.4)
, the tem-
plate structure (
3.5)
and the template structure mapped with four pharmacophore elements (two hydrogen-
bond acceptors [green] and two hydrophobic pharmacophore elements [cyan]).
signii cant afi nity. The alignment of pharmacophore elements in Figure 3.1 is in this case trivial as
all compounds in the series have the same molecular skeleton.
3.4.1 T
HE
I
NITIAL
P
HARMACOPHORE
M
ODEL
In order to develop a computer representation of the pharmacophore model, which also includes
information on the available space at important substituent positions (the last step in Figure 3.1), and
to extend the simplistic pharmacophore model described earlier to include representations of interactions
of pharmacophore elements with receptor sites, three substituted l avones (
3.2
-
3.4
) shown in Figure 3.2
were selected. Since small substituents in the 6-position increases the afi nity, compound
3.2
with
a bromo substituent in the 6-position was selected. Compound
3.3
with nitro groups in the 5- and
3
-positions was selected due to the favorable effect on the afi nity for small substituents in these
positions. Finally, compound
3.4
was selected as a representative of compounds in the available
series that carry a large substituent in the 3
′
-position but still display a reasonable receptor afi nity.
Since all three compounds have the same skeleton, they were for simplicity merged into a single
template molecule (
3.5
) displaying all the important features of
3.2
-
3.4
. Alternatively, each of
compounds
3.2
-
3.4
could have been analyzed by the computer program CATALYST for common
pharmacophore elements.
The template molecule
3.5
was used as input to the widely used computer program CATALYST
(Ac c el r ys Sof t wa r e I nc.), wh ich a na lyz es mole cu les i n t er m s of pha r m a cophor ic fe at u r es a nd d isplays
the pharmacophore elements as spheres. An advantage of this approach is that the pharmacophore
′