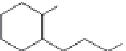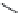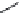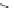Biomedical Engineering Reference
In-Depth Information
OH
O
O
CH
3
O
CH
3
H
3
C
H
3
C
+ 2 Fe
++
+ 2 H
+
+ 2 Fe
+++
H
3
C
CH
3
H
3
C
CH
3
O
O
n
n
OH
CH
3
O
CH
3
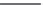
SCHEME 21.3
Oxidation of ubiquinol in the
bc
1
complex.
21.5.4.1 Naphtoquinones
The antimalarial naphtoquinones are developed from naturally occurring naphtoquinones such as,
lapachol (
21.18
) (Figure 21.11). The problem of fast metabolism, however, prevented the clinical
use. Among the several hundreds of napthoquinones synthesized and tested atovaquone (
21.18
)
was i nally selected for use. Atovaquone is assumed to bind to the ubiquinol oxidation pocket of the
parasite and thereby prevent the electron transfer. Model studies performed on the yeast
bc
1
complex
suggest that a hydrogen bond between the hydroxyl group of atovaquone and nitrogen of His181 of
yeast Rieske-protein and a hydrogen bond between Glu272 of
bc
1
complex via a water molecule
and one of the carbonyls of atovaquone stabilize the complex and thereby prevent transfer of the
electrons to the iron-sulfur complex. Replacement of Leu275 with the more bulky Phe275 as found
in bovine
bc
1
prevents the binding of atovaquone in the pocket (Figure 21.12). Similar atovaquone
only possesses a poor afi nity for human cytochrome
bc
1
.
Rapid development of resistance and a high rate of recrudescence necessitated the use of combi-
nation therapy. Proguanil (
21.29
) (refer to Figure 21.16)-atovaquone combination (Malarone
®
) is at
the present an effective therapy for multidrug resistant falciparum malaria. Unfortunately, the high
costs of this treatment limit its use.
O
CH
3
CH
3
O
Cl
OH
OH
O
O
21.19
21.18
FIGURE 21.11
Coni gurations of lapachol (
21.18
) and atovaquone (
21.19
).
Cytochrome
His181
Rieske protein
Leu275
Cl
N
HN
H
O
Glu272
O
O
O
OH
H
H
O
FIGURE 21.12
Suggested binding of atovaquone to the ubiquinol binding site.