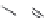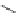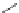Biomedical Engineering Reference
In-Depth Information
CH
3
NH
2
HN
N
O
CH
3
CH
3
NH
2
H
3
C
HN
O
N
CH
3
N
F
3
C
O
H
3
C
CH
3
N
+
O
N
S
CH
3
CH
3
CH
3
21.20
21.21
21.22
FIGURE 21.13
Coni gurations of methylene blue (
21.20
), primaquine (
21.21
), and tafenoquine (
21.22
).
21.5.5 D
RUGS
WITH
N
ONESTABLISHED
T
ARGETS
21.5.5.1 8-Aminoquinolines
Approximately 100 years ago, Paul Ehrlich (1854-1915) noticed a selective uptake and staining of
tissues with dyes such as methylene blue (
21.20
) (Figure 21.13). Based on the pioneering idea, at
that time, that this selective staining was caused by selective receptors for the dyes, he discovered
that methylene blue had antimalarial activity. Elaborating of this idea led to the development of the
8-aminoquinolines, among which primaquine (
21.21
) is the more important.
Primaquine remains the only drug approved for the cure of vivax malaria. The 8-aminoquino-
lines possess activity toward all stages of the parasite, including the hypnozoites in the liver and the
gametocytes in the blood. Killing of hypnozoites prevents relapse, which is caused by the activation
of hypnozoites resting in the liver. Relapse is pronounced for vivax malaria. Killing of gameto-
cytes prevents transmission. The ability to affect all stages reveals that the mechanism of action
of the 8-aminoquinolines must differ from that of the 4-aminoquinolines, which only affect para-
sites digesting hemoglobin (Section 21.5.1.1). Drawbacks of primaquine include a narrow therapeutic
window, a short half-life (4-6 h), which requires repeated administration for 14 days to achieve a
cure, and hemolysis and methemoglobin formation. The latter side effect is particularly pronounced
in patients with an inborn dei ciency of glucose-6-phosphate dehydrogenase, a genetic abnormality
common in areas where malaria is endemic. Structure-activity relationships (SARs) have revealed
that an appropriate substitution in the 2-position improved efi cacy and decreased general systemic
toxicity, a methyl group in the 4-position improved not only the therapeutic activity but also toxicity,
and that a phenoxy group in the 5-position decreased toxicity and maintained activity. The studies
led to synthesis of tafenoquine (
21.22
). The substituent in the 5-position provides tafenoquine with a
half-life of 2-3 weeks. A long half-life is essential for the development of a single-dose oral cure for
malaria.
The mechanism of action of the 8-aimonquinolines has not yet been established. It is suggested
that the compounds might affect the calcium homeostasis, affect the mitochondria by causing oxi-
dative stress, or act by a combination of these effects.
21.5.5.2 4-Quinolinemethanols
The quinolinemethanols might be considered as methanol substituted with 4-quinoline and an ali-
phatic substituent (Figure 21.14).
21.5.5.2.1 The Cinchona Alkaloids
The oldest representatives for the quinolinemethanols are (−)-quinine (
21.23
), (−)-cinchonidine
(
21.24
), (+)-quinidine (
21.25
), and (+)-cinchonine (
21.26
). All four alkaloids are isolated from the
bark of trees belonging to the genus
Cinchona
(Rubiaceae), often referred to as fever trees. Until
isolation of quinine in large scale in 1820, the crude bark was the only efi cient drug in Europe for