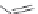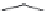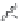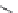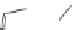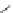Biomedical Engineering Reference
In-Depth Information
HO
Unselective opiates
R
H
OH
H
H
OMe
+
+
OH
N
+
N
R
N
R
O
O
O
O
OH
OH
OH
Morphine R = Me
Naltrexone R =
Diprenorphine R = Me
Naloxone R =
Buprenorphine R =
t
-Bu
Nalorphine R =
Selective opiates
R
H
OH
H
OH
H
OH
+
+
N
+
N
N
R
N
O
H
O
O
O
O
OH
OH
O
OMe
OH
R=H
7-Spiroindanyloxymorphone (SIOM)
Naltrindole (NTI)
β-Funaltrexamine (β-FNA)
NH
2
NH
2
+
Guanidinyl-NTI (gNTI)
R=
H
FIGURE 19.3
Chemical structures of classical unselective opiates (except morphine, which is m-selective)
based on the morphine scaffold and chemical structures of selective opiates. Morphine and b-FNA are m-selective
agonist and irreversible antagonist, respectively. SIOM and NTI are examples of d-selective antagonists,
whereas the introduction of charged guanidinium group converts NTI into the k-selective antagonist gNTI.
an agonist and the irreversible antagonist b-funaltrexamine (b-FNA). 7-Spiroindanyloxymorphone
(SIOM) and naltrindole (NTI) are examples of d-selective antagonists, and guanidinyl-NTI (gNTI)
represents a k-selective antagonist. Interestingly, gNTI was recently shown to have higher afi nity
toward HEK-293 cells expressing KOR together with DOR or MOR compared to cells expressing
only KOR, and the agonist effect of gNTI at the heterodimers KOR/DOR and KOR/MOR could be
blocked by antagonists selective against DOR and MOR, respectively. This underlines the impor-
tance of heterodimerization and the fact that gNTI is analgesic when injected into the spinal cord
but not when injected into the brain, could arise from different heteromeric populations.
The development of selective opiates has followed the “message-address” concept. This states
that the amino and the aromatic group in the morphine determine the activity (the “message”) of the
opiates, whereas the lipophilic region around the allylic alcohol confers selectivity (the “address”).
This is demonstrated by the conversion of NTI from a d-selective antagonist into the k-selective
antagonist gNTI by the introduction of charged guanidinium group. Already in the 1960s, a
3D-pharmacophore model was conceived stating the importance of the spatial placement of the
amine, the aromatic group, and the lipophilic region for ligand afi nity. The successive breakdown
of the morphine structure has led to a number of simpler nonopiate structures obeying this early and
simple 3D-pharmacophore model. This breakdown is shown schematically in Figure 19.4 dei ning
a structural classes of opioid receptor ligands developed over the last century.
Examples of these classes are m-selective agonist fentanyl (piperidine), ethyl-ketocyclazine
(benzomorphane), methadone (phenylpropylamine), and meperidine (piperidine) (Figure 19.5).
However, other structural classes have appeared, such as d-selective agonist SNC-80 and k-selective
agonist U50,488, and more recently several new scaffolds come from screening compound libraries
of the cloned opioid receptors including heteromeric combinations.