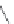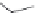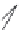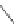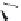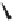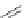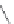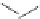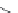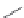Biomedical Engineering Reference
In-Depth Information
“Address”
H
N
+
OH
+
+
+
H
2
N
H
2
N
H
2
N
O
OH
“Message”
Piperidines
Benzomorphans
Morphinans
Morphine
+
+
+
+
+
H
3
N
H
2
N
H
2
N
H
2
N
H
2
N
O
O
O
Phenylpropylamines
Piperidines
Spiro[benzofuran]-
piperidines
Methylenoxy-
benzazocines
Morphines
FIGURE 19.4
The message-address concept of the development of opiates is shown schematically in the box.
The message region dei nes the activity of the compounds whereas the address region dei nes the selectivity
of the compounds. The structural development in the progressive simplii cation of the morphine scaffold via
morphinans and benzomorphans to piperidines, but also via benzazocines, spiropiperidines to piperidines and
phenylpropylamines.
O
H
+
N
N
N
N
Ar
N
N
O
R
O
OH
OMe
Fentanyl R = H; Ar = Ph
Sufentanil R = CH
2
OMe, Ar =
S
Ethyl-ketocyclazine (EKC)
SNC-80
O
Cl
N
N
O
O
Cl
N
O
N
Methadone
Meperidine
U50,488
FIGURE 19.5
Examples of different structural classes of opioid receptor ligands.
The dimerization of ligands is a popular strategy in medicinal chemistry to alter the pharma-
cological properties of a monomeric ligand. This strategy was advanced in the early 1980s by
Portoghese and coworkers using opiates. Initially, the idea was to develop such bivalent ligands
with a spacer of optimal length that would exhibit a potency that is greater than that derived from
the sum of its two monovalent pharmacophores. This would provide an evidence that the recep-
tors existed as dimers. One of the i rst series where compounds
19.1
(Figure 19.6,
n
= 0, 2, 4, 6, 8),
dimerizing a naltrexone analogue. The optimal spacer length was shown to be
n
= 4 giving the
highest activity.