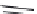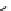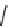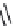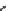Biomedical Engineering Reference
In-Depth Information
OCH
3
O
+
CH
3
CH
3
N
O
H
H
O
OH
N
OH
OH
N
O
O
H
2
N
HN
N
N
O
O
O
O
NH
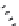
Muscimol (
15.22
)
THIP (
15.23
)
BMC (
15.24
)
SR 95531 (
15.25
)
OH
OH
OH
O
N
O
HN
HN
N
HN
O
4-PIOL (
15.26
)
Isoguvacine (
15.27
)
IAA (
15.28
)
OH
O
OH
OH
CH
3
P
O
O
HN
H
2
N
H
2
N
CACA (
15.29
)
CAMP (
15.30
)
TPMPA (
15.31
)
FIGURE 15.6
Structures of GABA
A
and GABA
C
ligands.
hypothesis originating from the bioactive conformation of muscimol, the partial GABA
A
agonist
4-PIOL (
15.26
), and on pharmacological data for an additional series of GABA
A
ligands, a simple
3D-pharmacophore model for the orthosteric GABA
A
receptor ligands has been developed. The
main features of this model are that the 3-hydroxyisoxazolol rings of muscimol and 4-PIOL do not
overlap in their proposed binding modes and that the two compounds interact with different con-
formations of an arginine residue located at the GABA
A
recognition site. The space surrounding
the ligands has been dei ned and the existence of a cavity of considerable dimensions in the vicinity
of the 4-position of the 3-hydroxyisoxazolol moiety in the structure of 4-PIOL has been identi-
i ed, whereas the corresponding position in muscimol is identii ed as “receptor essential volumes”
(Figure 15.7). Based on this model, a series of selective and highly potent competitive antagonists
have been developed including the compounds
15.32a-d
.
In contrast, structure-activity studies of ligands targeting the GABA
C
receptors have been very
limited.
cis
-4-Aminocrotonic acid (CACA [
15.29
]) (Figure 15.6) has been the key ligand for the
identii cation of the GABA
C
receptors. The compound is a moderately potent partial GABA
C
ago-
nist and inactive at GABA
A
receptors, but it has been shown to effect GABA transport as well. In
the search for selective GABA
C
receptor ligands, the folded conformation of CACA has been used
as a scaffold for new compounds such as
cis
-2-aminomethyl cyclopropane carboxylic acid (CAMP
[
15.30
]). (+)-CAMP has been reported to be a selective GABA
C
receptor agonist with potency in
the mid-micromolar range, displaying only weak activity on the GABA
A
receptors. Finally the
i rst antagonist capable of differentiating the GABA
C
receptors from both GABA
A
and GABA
B
receptors was TPMPA (
15.31
).