Biomedical Engineering Reference
In-Depth Information
ECL4
ECL4
ECL3
ECL2
Arg30
Asp404
Out
2Na
+
1
6
Leucine
5
4
2
3
8
7
9
10
11
12
Leu
TM1
TM6
Tyr268
Asp369
N
Arg5
C
(a)
(b)
6
1
3
8
(c)
(d)
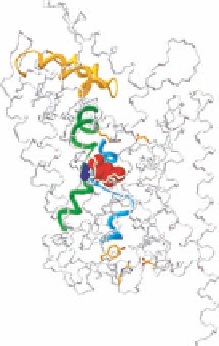
FIGURE 14.3
High-resolution structure of Na
+
/Cl
−
-coupled neurotransmitter transporter homologue (SLC6
family) from
A. aeolicus
(LeuT
Aa
). (a) Schematic representation of transmembrane topology. The binding
pocket for leucine and Na
+
is formed by TM1, TM3, TM6, and TM8 (TMs highlighted in distinct colors;
Na
+
denoted as blue dots). (b) View of LeuT
Aa
parallel to the membrane, highlighting important structural
features. The central parts of TM1 (green) and TM6 (light blue) are characterized by unwound breaks in the
helical structure. These breaks expose main carbonyl oxygen and nitrogen atoms for direct interaction with the
substrate (red). The two Na
+
ions (dark blue) also interact with the unwound part of TM1 and TM6 and have
a key role in stabilizing this structure and the leucine-binding site. Access to the substrate-binding site from
the external medium is predicted to be controlled by an external gate that in particular involves a charged pair
between Arg30 (TM1) and Asp404 (TM10) as well as conformational rearrangements of the ECL 4 (yellow),
which could form a lid that excludes the substrate-binding site from the extracellular space. A key residue in
the predicted intracellular gate is Tyr268 at the bottom of TM6. This residue is conserved in all transporters
of this class. Tyr268 interacts with Arg5 in the bottom of TM1, which again interacts with Asp369 at the bottom
of TM8, with both residues also highly conserved throughout the SLC6 family. (c) View parallel to the membrane.
(d) View from the extracellular side. The TMs forming the substrate-binding site are highlighted in distinct
colors. (From Gether, U. et al.,
Trends Pharmacol. Sci
., 27, 375, 2006. With permission.)
In agreement with this, recent experimental observations in the DAT in conjunction with computa-
tional simulations strongly support such a role of the interaction network and that the mechanism is
highly conserved among all SLC6 transporters (Figure 14.3).
A major reorganization on the intracellular side during translocation is further supported by
a marked increase in accessibility of cysteines introduced in the cytoplasmic half of TM5 of SERT to a
membrane permeant cysteine-reactive reagent in the presence of serotonin. The observations indicated
that when the transporter becomes inward-facing, the cytoplasmic half of TM5, which is occluded in
the LeuT structure, lines an aqueous pathway leading from the binding pocket to the cytoplasm.