Biomedical Engineering Reference
In-Depth Information
NT
Na
+
Cl
-
Na
+
NT
NT
Na
+
Na
+
Na
+
Cl
-
Na
+
Cl
-
NT
Na
+
Na
+
Cl
-
NT
Cl
-
Na
+
Na
+
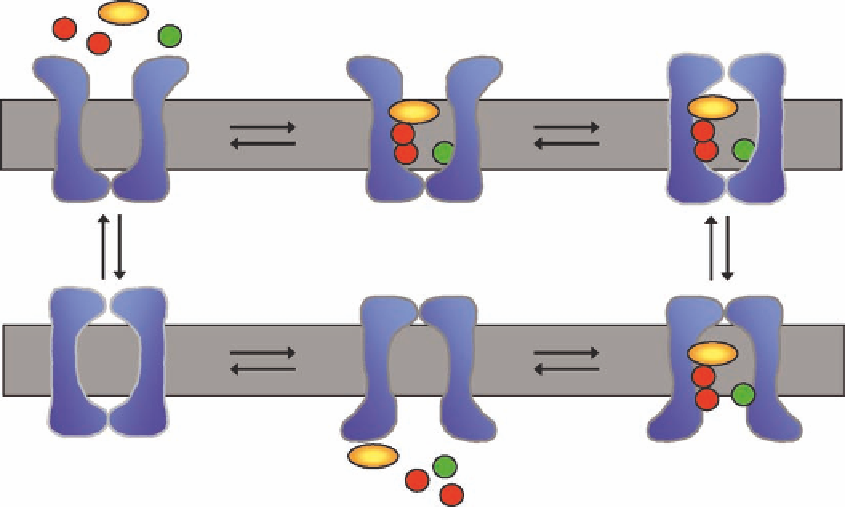
FIGURE 14.2
The alternating access model. The mechanism by which the neurotransmitter transporters
translocate the substrate from the extracellular environment to the cytosol can be explained by the alternating
access model. Without a neurotransmitter (NT) or ions (Na
+
, Cl
−
), the transporter resides in an outward facing
conformation where the neurotransmitter-binding site is only accessible to the external environment. Upon
binding of the solutes, the transporter undergoes a conformational change, i rst closing the outer gate excluding
access to the binding site and subsequently opening the inner gate, allowing access to the binding site from
the cytosol. The low Na
+
concentration in the cytosol allows the release of the Na
+
ions, which also causes a
release of its substrate. The release of solutes again closes the inner gate and opens the outer gate, making the
neurotransmitter transporter ready for another translocation cycle to occur. NT, neurotransmitter.
To accommodate shifts between outward- and inward-facing conformations it was suggested
that the transport process involves major movements of TM1 and TM6 relative to TM3 and TM8.
On the extracellular side, these movements were proposed to be controlled by an external gate that
involves residues in TM1, TM3, TM6, and TM10, with a charged pair between Arg30 (TM1) and
Asp404 (TM10) being of particular importance (Figure 14.3). Opening and closing of the external
gate are also likely to involve conformational rearrangements of the extracellular loops (ECLs).
ECL4 is especially interesting because it forms a lid that extends into the center of the transporter
and interacts with residues in, for example, TM1. In this way, ECL4 covers the substrate-binding
site from the outside without being in direct contact with the substrate (Figure 14.3). Thus, it is con-
ceivable that opening the transporter to the outside involves a major conformational rearrangement
of ECL4. This is in agreement with previous studies involving the engineering of Zn
2+
-binding sites
and application of the substituted-cysteine accessibility method in the corresponding part of the
DAT, SERT, and GABA transporter (GAT)-1.
The intracellular gate of LeuT
Aa
is predicted to comprise ~20 Å of ordered protein structure,
involving in particular the intracellular ends of TM1, TM6, and TM8. A key residue in the predicted
gate is Tyr268 at the cytoplasmic end of TM6, which is conserved in all transporters of this class
(Figure 14.3). The tyrosine is positioned below the substrate-binding site at the cytoplasmic surface
of the protein and forms a cation-
interaction with an arginine in the N-terminus just below TM1
that forms a salt bridge with an aspartate at the cytoplasmic end of TM8 (Asp369). A likely possibil-
ity would be that opening of the gate to the inside will require disruption of this set of interactions.
π